Imagine going to the doctor to get treatment for a persistent fever. Instead of giving you a pill or a shot, the doctor refers you to a special medical team which implants a tiny robot into your bloodstream. The robot detects the cause of your fever, travels to the appropriate system and provides a dose of medication directly to the infected area.
Surprisingly, we're not that far off from seeing devices like this actually used in medical procedures. They're called nanorobots and engineering teams around the world are working to design robots that will eventually be used to treat everything from hemophilia to cancer.
Bigger Isn't Always Better
In 1959, Richard Feynman, an engineer at CalTech, issued a challenge to engineers everywhere. He wanted someone to build a working motor that could fit within a cube 1/64th of an inch on each side. His hope was that by designing and building such a motor, engineers would develop new production methods that could be used in the emerging field of nanotechnology. In 1960, Bill McLellan claimed the prize, having built a working motor to the proper specifications. Feynman awarded the prize even though McLellan built the motor by hand without devising any new production methodologies.
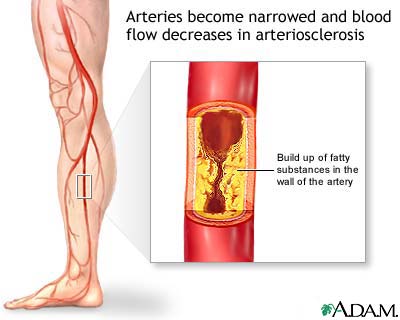
As you can imagine, the challenges facing engineers are daunting. A viable nanorobot has to be small and agile enough to navigate through the human circulatory system, an incredibly complex network of veins and arteries. The robot must also have the capacity to carry medication or miniature tools. Assuming the nanorobot isn't meant to stay in the patient forever, it also has to be able to make its way out of the host.
In this article, we'll learn about the potential applications of nanorobots, the various ways nanorobots will navigate and move through our bodies, the tools they will use to heal patients, the progress teams around the world have made so far and what theorists see in the future.
In the next section, we'll learn about the conditions and diseases nanorobots will treat in the future.
Advertisement