Geology
Geology is the study of the composition and physical properties of rocks, minerals, gems and other related earth materials, including diamonds and crystals. Scientists gain an understanding of the Earth's history by studying its composition.

Is Africa Splitting in Two? Really? Here's the Scoop

What Exactly Is the Eye of the Sahara, aka the Richat Structure?

The Driest Place on Earth: Chile's Atacama Desert

7 Power Crystals for Protection and Positive Energy

Carnelian Meaning: Healing Properties, Benefits, & Symbolism

Creating Crystal Grids: A Step-by-Step Guide

Velociraptor Alert: The Feathered Dinosaur Quiz

The Rockin' State Fossils Quiz

10 Extinct Hominids
Learn More
The notion of Africa splitting has the attention scientists and geologists worldwide, as the Great Rift Valley stretches and tears at the Earth's crust.
In the western Sahara Desert lies a natural wonder that has intrigued scientists and adventurers for centuries. Known as the Richat Structure — or, more commonly, the Eye of the Sahara — this massive geological formation resembles a giant eye.
By Marie Look
The Atacama Desert, situated in northern Chile, is not just any ordinary arid region. Spanning over 600 miles (965 km) along the Pacific Coast of South America, it is one of the most extreme landscapes on the planet. Thanks to certain oceanic conditions, there are areas that have received zero rainfall throughout recorded history, making the Atacama Desert the driest place on Earth.
By Marie Look
Advertisement
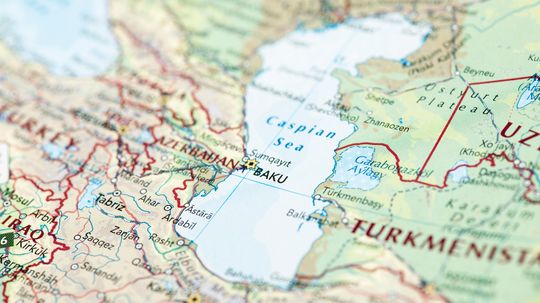
One of Earth's most interesting natural features, the Caspian Sea is the largest inland body of water on the planet, defying conventional classifications to be both a sea and a lake.
By Marie Look
Discover the deep allure of smoky quartz meaning. Explore its grounding properties and metaphysical benefits. Unveil the mystique today.
By HowStuffWorks
Unveil the profound obsidian meaning. Explore its spiritual, protective, and healing properties. Delve into the mystique today.
By HowStuffWorks
Explore the vibrant carnelian meaning. Uncover its energetic properties and spiritual significance. Embrace the power of carnelian today.
By HowStuffWorks
Advertisement
Discover Amazonite meaning: a soothing gemstone of balance. Explore its calming properties and spiritual significance. Dive into Amazonite's world.
By HowStuffWorks
Explore Aventurine meaning: a shimmering gem of luck and opportunity. Discover its healing properties and spiritual significance. Dive in now.
By HowStuffWorks
Discover Jade meaning: a revered gem of harmony and wisdom. Uncover its cultural significance and powerful metaphysical properties. Dive in now.
By HowStuffWorks
Uncover Pyrite meaning: a glittering symbol of wealth & protection. Explore its properties & spiritual significance. Delve into Pyrite's allure now.
By HowStuffWorks
Advertisement
Unveil Red Jasper Crystal Meaning: Grounding energy & vitality. Explore properties & spiritual significance. Dive into Red Jasper's world.
By HowStuffWorks
Explore Chakra Crystals: Balancing energy centers for holistic wellness. Discover properties & benefits of crystal healing. Dive in now.
By HowStuffWorks
Discover Crystals for Protection: Harness the power of protective stones. Explore their meanings and benefits. Enhance your energy shield today.
By HowStuffWorks
Explore Crystals for Love: Harness the energy of love-attracting stones. Discover meanings and benefits. Enhance your heart's resonance today.
By HowStuffWorks
Advertisement
Explore Crystal Grids: Amplify energy with sacred geometric arrangements. Learn about their uses and benefits. Elevate your intentions now.
By HowStuffWorks
Discover Clear Quartz meaning - a versatile crystal known for clarity & energy. Explore its spiritual uses & properties. Unlock insights now.
By HowStuffWorks
Explore Citrine Meaning - Unveiling Its Power and Significance. Discover the spiritual and healing properties of Citrine crystal.
By HowStuffWorks
Discover the profound Amethyst meaning: spiritual growth, healing, clarity. Explore its benefits & symbolism. Uncover Amethyst's wonders now.
By HowStuffWorks
Advertisement
Explore Labradorite Meaning: Mystical properties, spiritual insights, and transformation. Uncover the magic of Labradorite symbolism.
By HowStuffWorks
Discover Tigers Eye meaning: Courage, protection, and insight. Unveil the power of Tigers Eye symbolism. Explore its benefits now.
By HowStuffWorks
Unlock Moonstone Meaning: Intuition, cycles, and emotional balance. Delve into Moonstone symbolism. Discover its profound significance.
By HowStuffWorks
Birds are — quite literally — living dinosaurs. Our quiz will test your knowledge of the fluffy, downy and winged dinos of the bygone Mesozoic era, from little Microraptor to the enormous Yutyrannus.
By Mark Mancini
Advertisement
To honor their prehistoric pasts, most U.S. states have designated official state fossils, ranging from trilobites to dinosaurs. Take our quiz to learn more!
By Mark Mancini
Decades after the massive conflict, reminders of battles linger in pristine Pacific waters.