Electricity
Electricity is a major force of nature. In this section, you can learn how electricity works and what its potential uses are.
Brown Noise vs. White Noise: Which Is Best for Quality Sleep?

Can a sound wave kill you?

Can two cans and a string really be used to talk over a distance?

Delta-8 vs. Delta-9: Comparing Types of THC
Strong Bases: Properties, Applications and Examples

Comparing Strong Acids and Weak Acids

What do bugs have to do with forensic science?

5 Things You Didn't Know About Autopsies

Do a Person’s Fingerprints Change After Death?

How Alchemy Paved the Way for Chemistry

How did Nikola Tesla change the way we use energy?

Time May Not Exist, Say Some Physicists and Philosophers

Why Does Ice Stick to Your Fingers?
What if I forgot to remove a piercing before an MRI?
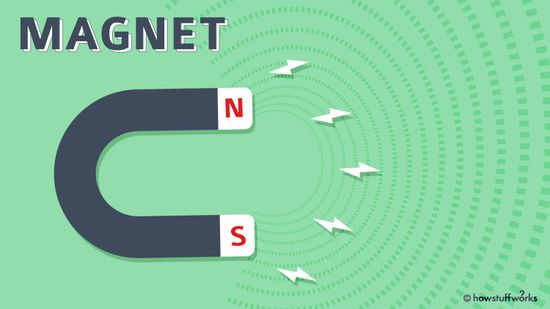
A Kid-friendly Introduction to Magnets and Magnetism

Deciphering 'Greater Than,' 'Less Than' and 'Equal To' Symbols

Getting a Handle on Fraction-to-Decimal Conversions

How to Make a Number Line for the Classroom

5 Hugely Fun Facts About Mass (Not Weight)

Antarctica's Spooky Cosmic Rays Might Shatter Physics As We Know It

Entropy: The Invisible Force That Brings Disorder to the Universe

The Demon Core: A Tale of Atomic Ambition and Tragic Fate

Half-Life Formula: Components and Applications

Could an 'X17 Particle' Hint at a Fifth Force in the Universe?

Why Are School Buses Yellow?

HowStuffWorks: How To Draw An Impossible Shape
What Are the Colors in the Visible Spectrum?
Learn More
Without gasoline, the world as we know it would grind to a screeching halt. The U.S. alone consumes well over a hundred billion gallons of gasoline per year. Learn all about this vital fuel.
Voltage is how we measure the difference in electric potential energy. Learn about what voltage is from this article.
Electricity completely surrounds us whether you're charging your cell phone or watching the sky light up during a violent thunderstorm. For most of us, modern life would be impossible without it, and the natural world relies on it.
Advertisement
Static electricity happens when there's an imbalance between negative and positive charges in an object. It's when those charges get released that we feel that infamous spark.
Scientists have figured out why some objects stick more to each other. And it's a very cool trick.
By Alia Hoyt
What happens when there's too much voltage? Learn about the difference between voltage surges and spikes from this article.
Thanks to the Faraday cage, we can control electricity and make it safer for our computers, cars and other inventions.
Advertisement
You want to learn how to read the electric power meter in your home to see if the meter reader sent by the electric company was accurate. This article will teach you how to read a power meter.







