When heavy, lung-damaging smog descends on cities like Los Angeles and Milan, it's natural to raise our fists and curse ozone. Ozone molecules, simply three oxygen atoms bound together, are extremely reactive and can cause real damage at ground level. But higher up, ozone is a beneficial and crucial component of Earth's atmosphere.
The stratosphere -- the layer of our atmosphere just above the one we breathe -- includes only a thin layer of ozone. There are about three ozone (O3) molecules for every 10 million air molecules, and this layer is thicker over the poles than the equator [source: NOAA]. It might seem insignificant compared to the depth of the rest of the atmosphere, but it does a very important job. It prevents much of the sun's ultraviolet-B (UV-B) light, from reaching the Earth. This UV light can cause skin cancer, cataracts and other disorders.
Advertisement
Ozone protects us from the sun by interacting with light. It's created when ultraviolet light hits oxygen molecules (O2) in the stratosphere, splitting the molecules into two atoms of oxygen (O). When this atom encounters another oxygen molecule, the two combine to make ozone (O3). Ultraviolet light also breaks ozone back down into an oxygen molecule and an oxygen atom. Check out this animation from NASA to see how this works.
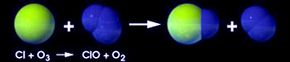
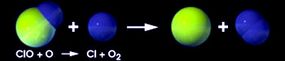
This process is called the ozone-oxygen cycle, and it converts UV radiation into heat, protecting the Earth. Other substances in the stratosphere, like chlorine, break the ozone back down into oxygen molecules and atoms. Usually, the building up and breaking down is a balanced process, but it can change according to seasons and because of natural events like volcanic eruptions.
But most scientists agree that human activity has caused an imbalance in the oxygen-ozone cycle that has led to a hole in the ozone layer over Antarctica. In this article, we'll find out what's causing the hole, whether we can create a patch, and what we can do to help stop the depletion of our critical UV protection.
So, how does ozone depletion happen in the first place?
Advertisement