Plastics are some of the most useful and moldable materials ever created by humankind. Yet one of their outstanding characteristics -- durability -- also makes them a potential long-term pollutant.
It doesn't take much to see the visual impact of discarded plastics. All around us, in cities and the countryside, plastic litter is evident in the form of old bottles, grocery bags, electronics packaging and much more. Those plastics aren't going away anytime soon.
Advertisement
That's because no natural processes decompose plastic. And as such, No one can honestly say they know for sure how long plastic debris lasts. Estimates range into hundreds and even thousands of years. That's because plastics are synthetic, petroleum-based polymers. Polymers are large chains of molecules, and in the case of plastics, they are just too gargantuan for microbes to even begin to attack.
Some manufacturers integrate additives into plastics to make them biodegrade in landfills and the environment. These organic additives attract bacteria, fungi and other microbes, which slowly acclimate to the plastic and break it down into organic molecules with various combinations acids and enzymes. But the majority of plastics don't have these additives, so they're nearly impervious to microbial attacks.
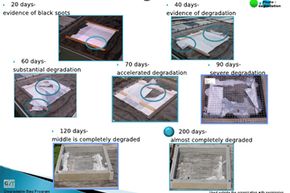
However, ultraviolet light can and does cause plastic to disintegrate, through a process called photodegradation. Photodegradation is the breakdown of complex materials into simpler ones due to light exposure.
The world might be a much cleaner place if all plastics would just vanish in sunlight. Keep reading and you'll see just how realistic this kind of plastics technology is.
Advertisement