In the last section, we looked at the simplest aerosol-can design, which uses compressed gas as a propellant. In the more popular system, the propellant is a liquefied gas. This means that the propellant will take liquid form when it is highly compressed, even if it is kept well above its boiling point.
Since the product is liquid at room temperature, it is simply poured in before the can is sealed. The propellant, on the other hand, must be pumped in under high pressure after the can is sealed. When the propellent is kept under high enough pressure, it doesn't have any room to expand into a gas. It stays in liquid form as long as the pressure is maintained. (This is the same principle used in a liquid propane grill.)
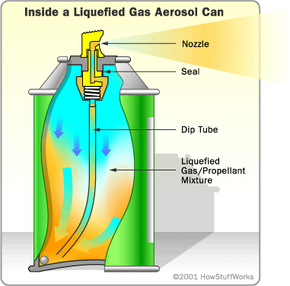
As you can see in the diagram below, the actual can design in this liquefied-gas system is exactly the same as in the compressed-gas system. But things work a little bit differently when you press down the button.
When the valve is open, the pressure on the liquid propellant is instantly reduced. With less pressure, it can begin to boil. Particles break free, forming a gas layer at the top of the can. This pressurized gas layer pushes the liquid product, as well as some of the liquid propellant, up the tube to the nozzle. Some cans, such as spray-paint cans, have a ball bearing inside. If you shake the can, the rattling ball bearing helps to mix up the propellant and the product, so the product is pushed out in a fine mist.
When the liquid flows through the nozzle, the propellant rapidly expands into gas. In some aerosol cans, this action helps to atomize the product, forming an extremely fine spray. In other designs, the evaporating propellant forms bubbles in the product, creating a foam. The consistency of the expelled product depends on several factors, including:
- The chemical makeup of the propellant and product
- The ratio of propellant to product
- The pressure of the propellant
- The size and shape of the valve system
Manufacturers are able to produce a wide variety of aerosol devices by configuring these elements in different combinations. But whether the can shoots out foamy whipped cream, thick shaving gel or a fine mist of deodorant, the basic mechanism at work is the same: One fluid pushes another.
To learn more about aerosol cans, as well as the chemicals used inside them, check out the links on the next page.