Optics
Optics is the study of the properties and behavior of light. In this section you can learn about everything from holograms to lasers and lenses.
Brown Noise vs. White Noise: Which Is Best for Quality Sleep?

Can a sound wave kill you?

Can two cans and a string really be used to talk over a distance?

Delta-8 vs. Delta-9: Comparing Types of THC
Strong Bases: Properties, Applications and Examples

Comparing Strong Acids and Weak Acids

How Electricity Works

How Faraday Cages Work

How Gasoline Works

What do bugs have to do with forensic science?

5 Things You Didn't Know About Autopsies

Do a Person’s Fingerprints Change After Death?

How Alchemy Paved the Way for Chemistry

How did Nikola Tesla change the way we use energy?

Time May Not Exist, Say Some Physicists and Philosophers

Why Does Ice Stick to Your Fingers?
What if I forgot to remove a piercing before an MRI?
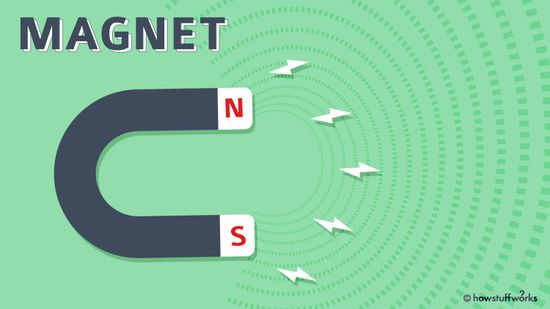
A Kid-friendly Introduction to Magnets and Magnetism

Deciphering 'Greater Than,' 'Less Than' and 'Equal To' Symbols

Getting a Handle on Fraction-to-Decimal Conversions

How to Make a Number Line for the Classroom

5 Hugely Fun Facts About Mass (Not Weight)

Antarctica's Spooky Cosmic Rays Might Shatter Physics As We Know It

Entropy: The Invisible Force That Brings Disorder to the Universe

The Demon Core: A Tale of Atomic Ambition and Tragic Fate

Half-Life Formula: Components and Applications

Could an 'X17 Particle' Hint at a Fifth Force in the Universe?
Learn More
The iconic "school bus yellow" was the invention of an educator named Frank Cyr. But if yellow is so good for visibility, why don't all fire trucks use it too?
By Dave Roos
It’s true: In 6 easy steps, you too can draw an impossible shape.
All colors that you see fall into the visible light spectrum. Learn about the colors in the visible light spectrum in this article.
By Sascha Bos
Advertisement
We first reported on the possibility of an invisibility cloak last year. Now a different invisibility cloak is making the news -- one that uses metamaterials to redirect light away from the wearer.
Imagine wearing a T-shirt with lettering on it while brushing your teeth. Why are the letters on the T-shirt reversed in the mirror, while your head appears right side up?
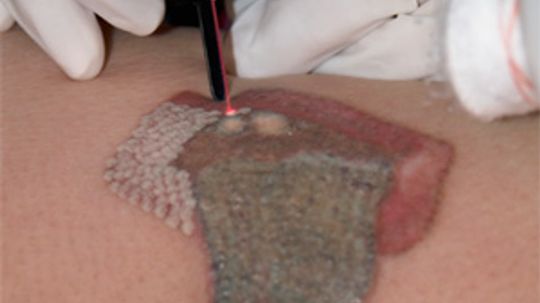
Lasers are used in dental drills, eye surgery and even tattoo removal. But what exactly is a laser? There are numerous types, but all lasers work basically the same way. Learn how they generate such concentrated beams of light.
We all have favorite colors. But have you ever considered why you like one color more than another?
Advertisement
You're driving down the road on a sunny day, and you see a puddle of water coming up. You look again and it's gone! What happened? You’ll be able to answer that question if you read our miraculous mirage article.
By Tom Harris
The Diamond synchrotron is a massive facility that houses a beam of light 10 billion times brighter than the sun. But is that all it does?
A venerable work of art hangs lifeless in a museum, the once brilliant scene dulled by centuries of dirt and grime. Can laser analysis and modern art restoration techniques save the masterpiece?
If you want to see a hologram, you don't have to look much farther than your wallet. But the most impressive holograms are large scale and illuminated with lasers or displayed in a darkened room with carefully directed lighting. Learn how a hologram, light and your brain work together make clear, 3-D images.
Advertisement
Unlike the cheap microscopes you peered into in school, these advanced instruments can breathe rich detail into the tiny world around us, including the world of nanotechnology.
For centuries, curious observers have probed the heavens with the aid of telescopes. Today, both amateur and professional scopes magnify images in a variety of ways.
The human eye misses a lot -- enter the incredible world of the microscopic! Explore how a light microscope works.
Some of the brightest minds in history have focused their intellects on the subject of light. Einstein even tried to imagine riding on a beam of light. We won't get that crazy, but we will shine a light on everything scientists have found so far.
Advertisement
Your eyes aren't playing tricks on you. Those mountains way off in the distance really do look blue, and it's because of how light wavelengths scatter in the atmosphere.
By Mark Mancini
Just how far can the human eye see? There's no exact formula to figuring it out, but we do have an idea.
I have a thin piece of plastic mounted on the back window of my RV. It magnifies things so I can see better when I'm backing up. How can such a thin piece of plastic magnify things? A regular glass magnifying lens would have to be curved on both sides and much thicker.
Just about everyone has seen a television show or movie in which a criminal suspect is questioned while detectives watch from behind a one-way mirror. How does a piece of glass manage to reflect light from one side while remaining clear on the other?
Advertisement
Kaleidoscopes have been fascinating people since the early 19th century. Whether you think of kaleidoscopes as toys or as works of art, no matter how often you look inside, you'll never see the same thing twice.
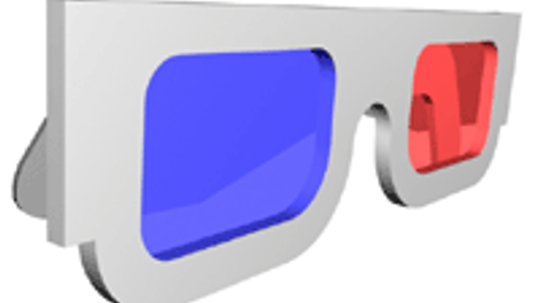
NASA's Mars rovers are sending 3-D images to Earth, so we can see depth and texture on the Martian surface. And how do we see this depth and texture? 3-D glasses, of course! Check out how they work.
An invisibility cloak seems perfectly believable in the magical world of Harry Potter, but in the real world, it's impossible, right? Not so fast.
What if there are colors within the visible spectrum that our brains can't perceive? In fact, there are. They're called impossible colors. But some researchers think they've discovered a way to see the impossible.
By Dave Roos
Advertisement
When speed is everything and light marks the universe's speed limit, lasers are bound to be the answer. At least, that's what NASA and a bunch of Wall Street types are betting on.
Cosmological redshift: sounds like the latest blockbuster coming to a theater near you, doesn't it? In reality, it has to do with how light itself travels -- and understanding how it works is essential to advanced space telescope technology.