Orbiting the nucleus are the electrons, ultra-light particles with negative charges. Electrons facilitate chemical bonding — and their movements can produce a little thing called electricity. Protons are no less important. For one thing, they help scientists tell the elements apart.
You might have noticed that in most versions of the periodic table, each square has a little number printed in its upper righthand corner above the element symbol. That figure is known as the atomic number. It tells the reader how many protons are in the atomic nucleus of a particular element. For example, oxygen's atomic number is eight. Every oxygen atom in the universe has a nucleus with exactly eight protons; no more, no less.
Without this very specific arrangement of particles, oxygen wouldn't be oxygen. Each element's atomic number — including oxygen's — is totally unique. No two elements can have the same atomic number. No other element has eight protons per nucleus. By counting the number of protons, you can identify an atom. Just as oxygen atoms will always have eight protons, nitrogen atoms invariably come with seven. It's that simple.
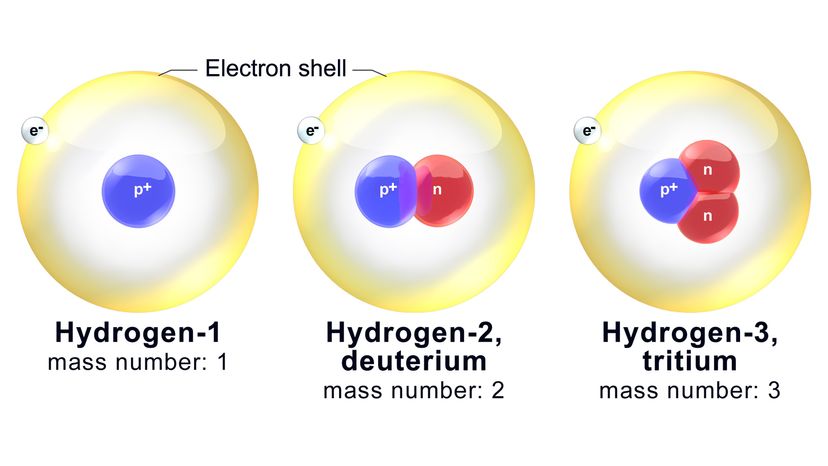
Neutrons do not follow suit. The nucleus in an oxygen atom is guaranteed to harbor eight protons (as we've established). However, it might also contain anywhere from four to 20 neutrons. Isotopes are variants of the same element that have different numbers of neutrons (and thus potentially different physical properties). They do, however, tend to have the same chemical properties.
Now, each isotope is named on the basis of its mass number, which is the total combined number of neutrons and protons in an atom. For example, one of the better-known oxygen isotopes is called oxygen-18 (O-18). It's got the standard eight protons plus 10 neutrons.
Ergo, the mass number of O-18 is — you guessed it — 18. A related isotope, oxygen-17 (O-17), has one fewer neutron in the nucleus. O-16, then, has the same number of protons and neutrons: eight. Among this trio, O-16 and O-17 are the lighter isotopes, and O-16 is also the most abundant isotope of the three.