On Earth, we have a constant supply of fresh air. We breathe in oxygen and breathe out carbon dioxide. These gases get recycled by plants through the process of photosynthesis. The plants take in carbon dioxide and release oxygen. It's a wonderful cycle on a vast scale. But what happens in the tiny, confined cabins of spacecraft, like the space shuttle or space stations?
Most spacecraft carry their own supply of oxygen with them and may have one backup system. However, the missions of these spacecraft last a short time, on the order of days to two weeks. In contrast, the International Space Station (ISS) was designed for long-term spaceflight and has been in orbit since 1998. So how is oxygen made aboard the ISS? It's handled in one of three ways, using oxygen generators, pressurized oxygen tanks or solid fuel oxygen generators (also called oxygen candles).
Advertisement
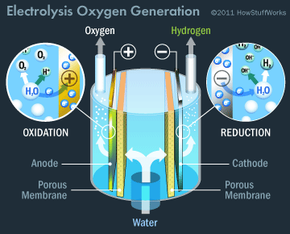
The primary method is accomplished by the oxygen generators: the Russian-made Elektron and the U.S. Environmental Control and Life Support System (ECLSS). The Elektron is located in the service module (Zvezda) and the ECLSS is located in the Destiny laboratory module. These devices make oxygen from water by a process called electrolysis, during which an electric current passes through water from one positively-charged electrode called an anode to another negatively-charged electrode called a cathode. There's a small concentration of salt in the water to conduct electricity because water itself is a poor electrical conductor. In the process, water gets split into hydrogen gas and oxygen gas. Here's how the chemistry of the process works:
- At the cathode, a type of reaction called reduction occurs. Electrons (e-) from the cathode combine with the water (H2O) to make hydrogen gas (H2) and hydroxide ions (OH-): 2H2O (l) + 2e- ->H2 (g) + 2 OH- (aq).
- At the anode, a type of reaction called oxidation occurs. Electrons get removed from water and flow into the anode. Removing the electrons from water yields oxygen gas (O2) and hydrogen ions (H+): 2H2O (l) -> O2 (g) + 4 e- + 4 H+
The electricity is generated by the station's solar panels and supplied to the oxygen generators through the station's power grid. The water gets delivered to the station from Earth by Progress supply ships and the space shuttle. Water also gets reclaimed by condensers that remove water vapor from the cabin air (astronauts exhale water vapor). Finally, water can be recycled from the astronauts' urine by the ECLSS unit. The hydrogen gas made in the electrolysis process gets vented into space and the oxygen gas is circulated into the cabin air.
Now let's look at the other ways by which the ISS makes oxygen.
Advertisement