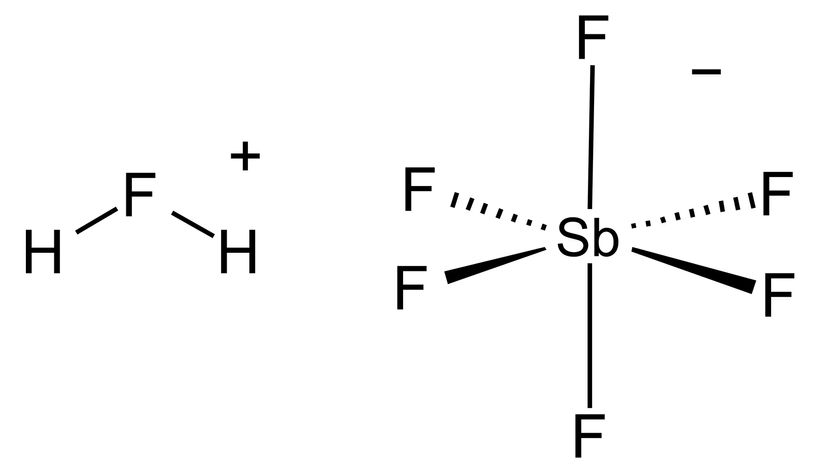
The chemical realm is vast, and while fluoroantimonic acid has its revered status, there's an array of other fascinating acids that deserve a mention. Let's explore the unique attributes of these acids, diving into their strengths, weaknesses and roles in various processes.
Carborane Acids
These are among the strongest of the strong acids, next only to fluoroantimonic acid. Their unique molecular structure, backed by the American Chemical Society's research, helps them maintain their potency.
What sets them apart is their weak bond with hydrogen ions, making them less corrosive than fluoroantimonic acid.
Magic Acid
It sounds fantastical, but magic acid is very real! It's formed by mixing fluorosulfuric acid (HSO₃F) and antimony pentafluoride (SbF₅). This acid completely dissociates in an aqueous solution, releasing a high concentration of hydrogen ions.
Nitric Acid, Phosphoric Acid and Perchloric Acid
These are examples of strong acids that completely dissociate when dissolved in water. While they may not match the extreme acidity of fluoroantimonic acid, they're essential in industries, ranging from fertilizer production to rocket propellant.
Benzoic Acid and Oxalic Acid
These weak acids don't completely dissociate in water. However, they play crucial roles in our daily lives, from food preservation to cleaning agents.
Hydronium Ion
All acids, when dissolved in water, produce this positively charged ion. It's the real culprit behind the acidic properties of a solution.
This article was updated in conjunction with AI technology, then fact-checked and edited by a HowStuffWorks editor.