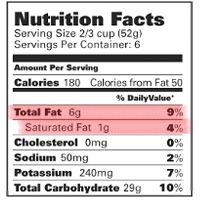
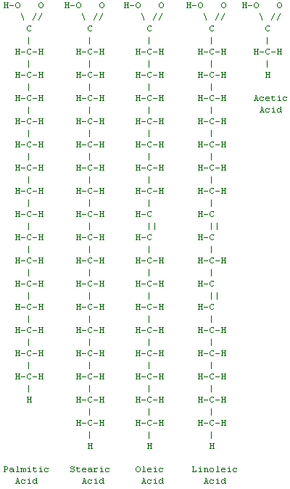
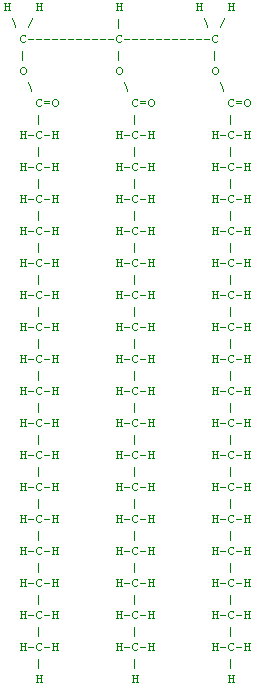
Between the food commercials you see on TV every day and the many nutrition bulletins and reports you hear about on the news every night, you get a huge amount of information about the fats that you eat. For example, you have probably heard of the following terms:
- Saturated fat
- Unsaturated fat
- Polyunsaturated fat
- Mono-unsaturated fat
- Fatty acids
- Essential fatty acids
- Trans fatty acids
- Omega-3 and omega-6 fatty acids
- Partially hydrogenated fat
Have you ever wondered what it all means, or why it matters? Why can't we just eat, drink and be merry? In this article, you'll find out exactly what these terms mean and how the various forms of fat you find in foods affect your body. But first, let's find out what we're talking about in practical terms.
Advertisement
We see pure fats in three places at the grocery store:
- In the vegetable oil aisle you see oils created from different seeds and nuts. There is corn oil, safflower oil, peanut oil, canola oil, olive oil... All seeds and nuts contain some amount of oil, because oil is a very good way to store energy. By the way, the only difference between oil and fat is whether or not it is a solid at room temperature.
- In the meat aisle, you can look at different cuts of meat and see them outlined by a layer of white, solid fat created by the animal to store energy.
- In the dairy aisle you see butter and margarine -- fat made from cream or vegetable oils, respectively.
The rest of the grocery store is, of course, filled with fats and oils, although they are less obvious. Potato chips and french fries are cooked in oil, cookies and cakes contain fats and oils, and so on. This is how we come to eat the fat we need every day. And we do need fat -- as you will learn in this article, there are certain fats that we must have to survive.
So what are these fats and oils really made of? Well, if you really want to understand fat you need to study a little bit of chemistry. To talk about fat, we need to start by talking about fatty acids.
Advertisement