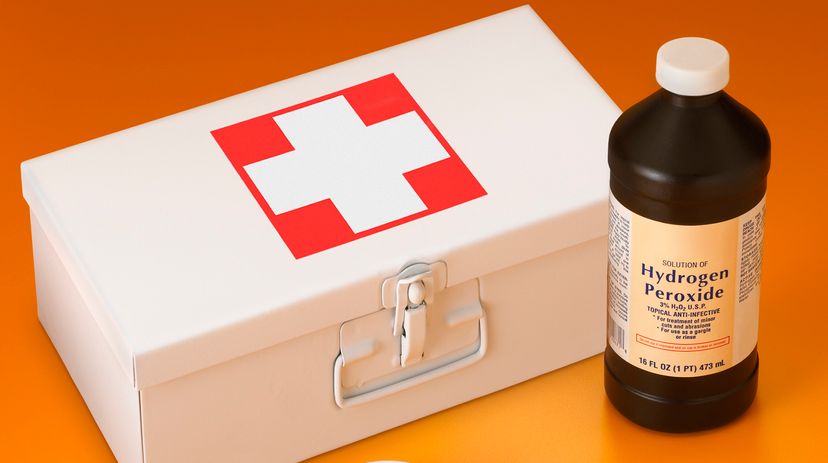
Hydrogen peroxide (H2O2) is a common bleaching agent that you can buy at the drugstore. What you are buying is a 3 percent solution, meaning the bottle contains 97 percent water and 3 percent hydrogen peroxide. Most people use it as an antiseptic.
It turns out that hydrogen peroxide is not very good as an antiseptic, but it is not bad for washing cuts and scrapes, and the foaming looks cool. So why does hydrogen peroxide bubble? Let's dive into the science behind the foamy display.
Advertisement