Key Takeaways
- Niels Bohr's model, which depicted electrons orbiting the nucleus like planets around the sun, was awarded a Nobel Prize in 1922 despite being technically incorrect.
- Arnold Sommerfeld enhanced the Bohr model in 1916 with elliptical orbits.
- In spite of its inaccuracies, the Bohr model remains a fundamental teaching tool in introducing the concept of atoms.
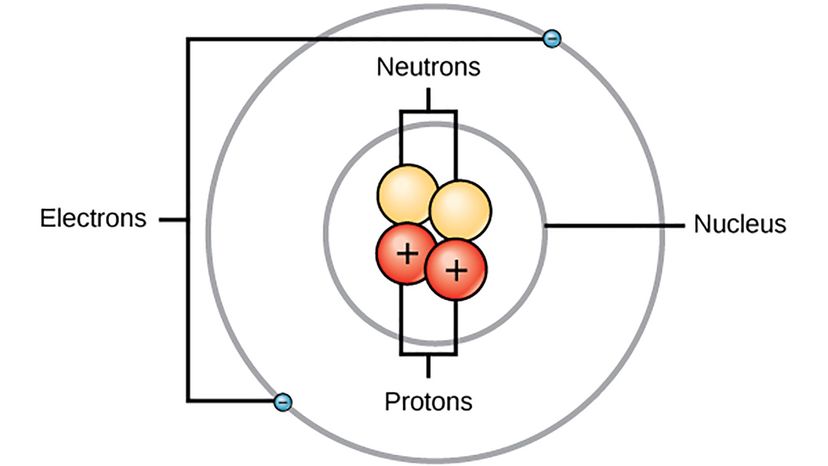
You can search for a picture of an atom on the internet and you'll find one, even though nobody's actually seen an atom before. But we've got an estimation of what a single atom looks like because of the work of a bunch of different scientists like Danish physicist Niels Bohr.
Atoms are the building blocks of matter — a single atom of any individual element is the most basic entity in nature that still abides by the rules of physics we can observe in everyday life (the subatomic particles that make up atoms have their own special rules). Scientists suspected atoms existed for a long time before they could conceptualize their structure — even the ancient Greeks figured the matter of the universe was made up of components so small they couldn't be broken down into anything smaller, and they called these fundamental units atomos, which means "undivided." By the end of the 19th century, it was understood that chemical substances could be broken down into atoms, which were very small and atoms of different elements had a predictable weight.
Advertisement
But then, in 1897, British physicist J.J. Thomson discovered electrons — negatively-charged particles inside the atoms everyone had spent the better part of a century believing were entirely indivisible — as the smallest things that existed. Thomson just hypothesized that electrons existed, but he couldn't work out exactly how electrons fit into an atom. His best guess was the "plum pudding model," which depicted the atom as a positively-charged pie studded with negatively-charged areas scattered throughout like fruit in an old-timey dessert.
"Electrons were found to be negative electric, and all with the same mass and very small compared with atoms," says Dudley Herschbach, a Harvard chemist who shared the Nobel Prize in Chemistry in 1986 for his "contributions concerning the dynamics of chemical elementary processes," in an email. "Ernest Rutherford discovered the nucleus in 1911. Nuclei were positive electric, with various masses but much larger than electrons, yet very small in size."
Advertisement


