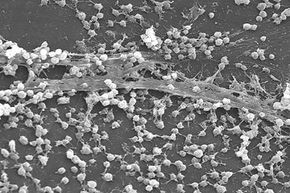
At first, it's not clear what dental plaque, the persistent slime in your shower drain and a slippery submerged rock have in common besides the fact that they can be a headache — or toothache — to remove. To the naked eye, it's nearly impossible to see what's responsible for these lined surfaces.
If you look closer, with the help of a microscope, you'll realize these slimy aggregations are anything but dull. Each biofilm consists of tiny communities of diverse living microorganisms bound together in a thick adhesive matrix. Who would've guessed the grimy buildup in your toilet bowl is a complex clump of living, communicating cells?
Advertisement
Though Antoni van Leeuwenhoek, the discoverer of bacteria, described similar formations when he studied his own dental plaque in the 17th century, it wasn't until the 20th century that scientists had the tools they needed to take a closer look at how the structures develop [sources: Montana State University CBE, Costerton and Wilson].
These colonies, also called biofilms, form when single microorganisms attach to a hydrated surface and undergo a "lifestyle switch," giving up life as a single cell to live on a surface in an adhesive cell matrix with other microorganisms [source: Lemon et al.]. Some definitions state that biofilm cells "irreversibly attach" to a surface, meaning gentle rinsing can't remove them [source: Donlan].
But why should we care about biofilms?
For starters, they can attach to both living and nonliving surfaces (including humans), create problems in the medical field, alter industrial production practices and even contribute to environmental cleanup. In addition, some researchers estimate that biofilms constitute more than half of the world's biomass [sources: Montana State University CBE; Sturman]. Biofilms are so abundant it's surprising that we don't notice them more.
Advertisement