Don't feel stupid if you've ever seen a news story about a terrible drought, then turned to your computer to see your pretty ocean beach screensaver and thought, "Why don't they just use that?"
Of course, within a moment, you probably made a few points to yourself. One, the sea is salty. Two, salty water isn't so great for drinking or growing plants. Three, you can't just take the salt out of water, just like you couldn't dissolve the sugar out of your tea. Or can you?
Advertisement
Reverse osmosis is one of the processes that makes desalination (or removing salt from seawater) possible. Beyond that, reverse osmosis is used for recycling, wastewater treatment, and can even produce energy.
Water issues have become an extremely pressing global threat. With climate change comes unprecedented environmental impacts: torrential flooding in some areas, droughts in others, rising and falling sea levels. Add to that the threat of overpopulation — and the demand and pollution a swelling population brings — and water becomes one of the paramount environmental issues to watch for in the next generation.
Water treatment plants and systems are now adapting reverse osmosis to address some of these concerns. In Perth, Australia (notably dry and arid, yet surrounded by sea), nearly 17 percent of the area's drinking water is desalinated sea water that comes from a reverse osmosis plant [source: ExcelCalcs]. Worldwide, there are now over 22,757 desalination plants in the world, according to the International Desalination Association.
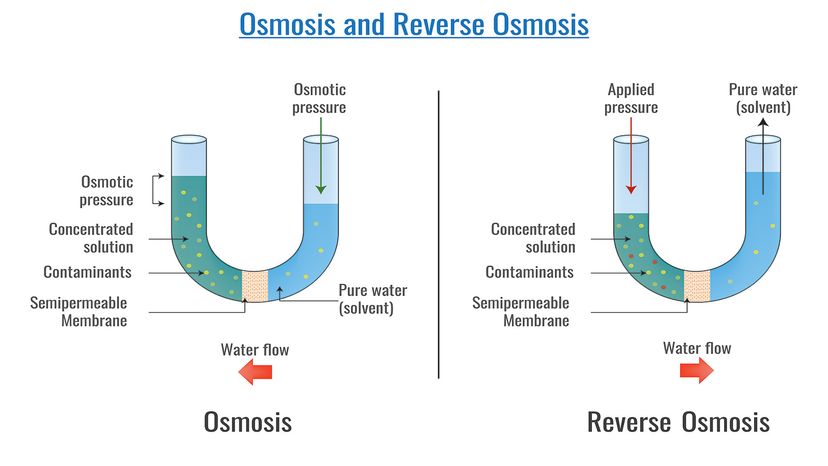
But while knowing that using a reverse osmosis membrane to convert seawater to drinking water is useful, what we really need to understand is how the heck the process occurs. Assuming that you have a fairly good grasp on the definition of "reverse," we better start by taking a look at how osmosis works before we put the two together.
Advertisement