Chemistry of Plastics
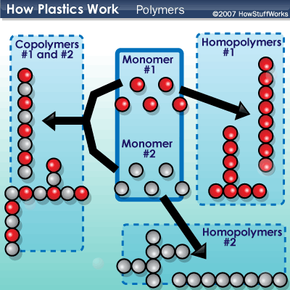
All plastics are polymers, but not all polymers are plastics. Some familiar nonplastic polymers include starches (polymers of sugars), proteins (polymers of amino acids) and DNA (polymers of nucleotides -- see How DNA Works). The simplified diagram below shows the relationship between monomers and polymers. Identical monomers can combine with each other to form homopolymers, which can be straight or branched chains. Different monomers may combine together to form copolymers, which also may be branched or straight.
The chemical properties of a polymer depend on:
Advertisement
- The type of monomer or monomers that make up the polymer. The chemical properties of homopolymer 1 are different from those of homopolymer 2 or the copolymers.
- The arrangement of monomers within the polymer. The chemical properties of the straight polymers are different from those of the branched polymers.
The monomers that are found in many plastics include organic compounds like ethylene, propylene, styrene, phenol, formaldehyde, ethylene glycol, vinyl chloride and acetonitrile (we'll examine many of these as we discuss various plastics). Because there are so many different monomers that can combine in many different ways, we can make many kinds of plastics.