Laser light has the following properties:
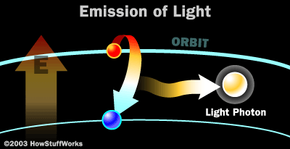
- The light released is monochromatic. It contains one specific wavelength of light (one specific color). The wavelength of light is determined by the amount of energy released when the electron drops to a lower orbit.
- The light released is coherent. It is “organized” — each photon moves in step with the others. This means that all of the photons have wave fronts that launch in unison.
- The light is very directional. A laser light has a very tight beam and is very strong and concentrated. A flashlight, on the other hand, releases light in many directions, and the light is very weak and diffuse.
Stimulated Emission
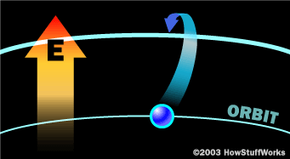
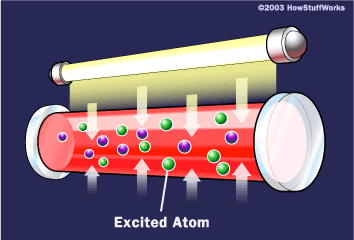
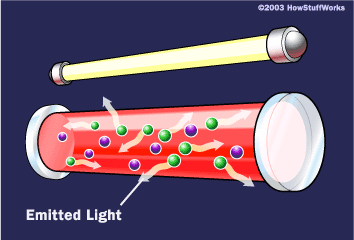
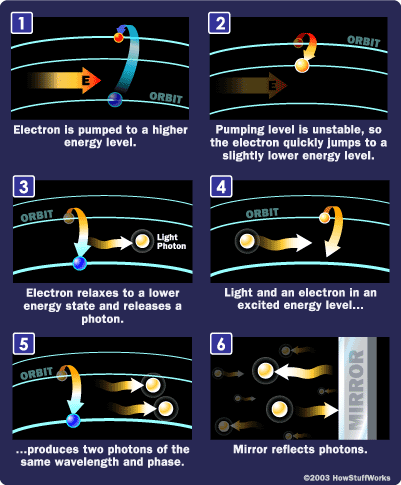
To make these three properties occur takes something called stimulated emission. This does not occur in your ordinary flashlight — in a flashlight, all of the atoms release their photons randomly. In stimulated emission, photon emission is organized.
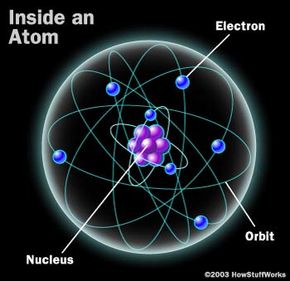
The photon that any atom releases has a certain wavelength that is dependent on the energy difference between the excited state and the ground state.
If this photon (possessing a certain energy and phase) should encounter another atom that has an electron in the same excited state, stimulated emission can occur. The first photon can stimulate or induce atomic emission such that the subsequent emitted photon (from the second atom) vibrates with the same frequency and direction as the incoming photon.
Mirrors
The other key to a laser is a pair of mirrors, one at each end of the lasing medium.
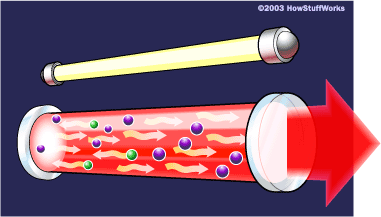
Photons, with a very specific wavelength and phase, reflect off the mirrors to travel back and forth through the lasing medium. In the process, they stimulate other electrons to make the downward energy jump and can cause the emission of more photons of the same wavelength and phase.
A cascade effect occurs, and soon we have propagated many, many photons of the same wavelength and phase. The mirror at one end of the laser is "half-silvered," meaning it reflects some light and lets some light through. The light that makes it through is the laser light.
You can see all of these components in the figures in the next section, which illustrate how a simple ruby laser works.