Incoming!
You jump to your feet at the slightest murmur of an attack. It's dark inside the bunker, and everywhere you look is blackness. Shells pound the ground no more than 50 meters in front of your position, rattling the fillings loose in your skull. Quickly you fumble in the darkness, looking for your rifle and helmet, but there's something odd about this attack. There's no explosion flash.
Advertisement
As you scramble to your position, the pounding stops and a low hissing fills the air, something you've never heard. Rifle in hand, you creep to the opening of your foxhole and peer out between two sandbags.
Your eyes begin to water as you try to focus on the scene in front of you. The clear, starry night fades as a creeping yellow fog slowly begins to consume your view.
To your left, soldiers in the bunker closest to the impact zones shout, "What's that smell?" You can make out a few hunched over at the waist, while several more frantically wave their hands in front of their faces.
The yellow fog creeps into your bunker, and you begin to lose your bearing. The sounds of men spitting and sneezing fill your ears. The air grows heavy, and the pungent garlic smell worsens. Panic sets in. You start to become dizzy from the heavy breathing, and your throat burns ever so slightly. You're in trouble.
Slowly the smell subsides, and the gas cloud dissipates. Everything around you swims into focus, and things settle down. Thankfully, you're breathing more easily and beginning to relax. You feel better now.
"No worries. It was just a smoke screen," you think.
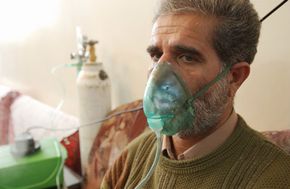
You're alive, having just survived your first mustard gas attack. Little do you know the worst is yet to come.
This scenario is what the first soldiers who experienced a mustard gas poisoning attack in World War I might have gone through. In this article, we'll learn about the horrendous mustard gas effects on soldiers and civilians during wartime. Read on and find out if you survived the gas attack, or what your fate might have been as we learn how mustard gas works.
Advertisement