A good example of a way in which humans take advantage of electronegativity everyday is Teflon, the polymer polytetrafluoroethylene (PTFE), which can coat a pan to keep your scrambled eggs from sticking to it. This polymer is a long chain of carbon-on-carbon bonds, where each internal carbon atom also has two fluorine atoms bonded to it. Of all the elements, fluorine is the most electronegative, so the bonding electrons are being held tightly to the fluorine atoms.
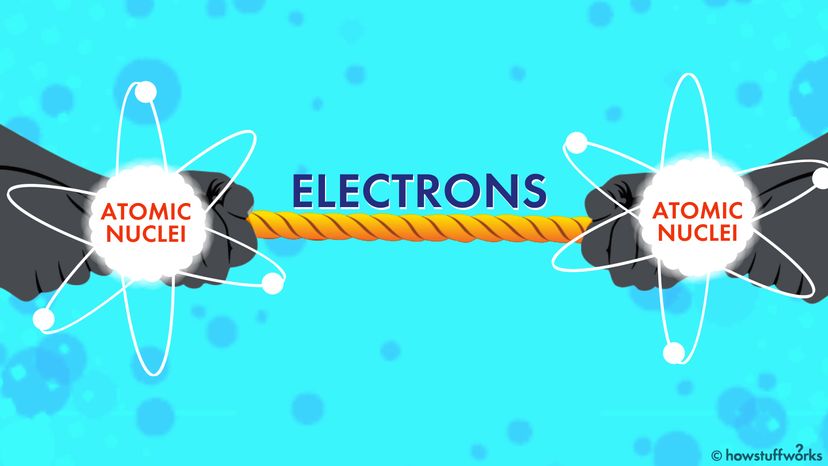
Molecules can be attracted to each other through special interactions, like London dispersion forces. These forces are created when the constantly moving electrons in a molecule are pulled to one area of the molecule, creating spots in the molecule that are more negatively charged and others that are more positively charged.
In the specific case of Teflon, because fluorine is so electronegative, the nuclei in its atoms minimize the amount of electron movement — the fluorine atom is so attractive to the electrons that they rarely want to hang out around the carbon nuclei at all. This means the electron motion that would create attractive London dispersion forces is nullified, which results in the "nonstick" characteristics of Teflon.
Electronegativity also plays into the creation of pharmaceuticals:
"Many drugs are small molecules, and they are designed to interact with certain proteins in the body that have specific functions," says Ferreira. "These interactions are based on the physical shape of the molecule to precisely fit in the protein's receptor shape — think of a key fitting into a lock. These intermolecular interactions can be based on electrostatic forces, and therefore one could design drugs where the electronic nature is "tuned" at specific atoms based on their electronegativity to maximize the efficacy of the interaction."
So, next time you drink a glass of water or make a grilled cheese sandwich or take your medicine, thank chemistry for making every element a little bit different — and some more attractive than others.