Have you ever heard of HeLa cells? They've been around for more than 60 years, but unless you're a medical researcher, the name probably didn't crop up on your radar until recently, if at all. In the past decade or so, countless articles -- and one New York Times bestselling book -- have been written about them.
But what's a HeLa cell? It's a line, or population, of cells, taken from a person and used in scientific research. Cell lines are often named after the people from whom they were originally derived, and HeLa comes from the first two letters in the name Henrietta Lacks. Cell lines are used in all kinds of ways, such as studying the effects of diseases or developing medications and vaccines, and play an invaluable role in medicine today.
Advertisement
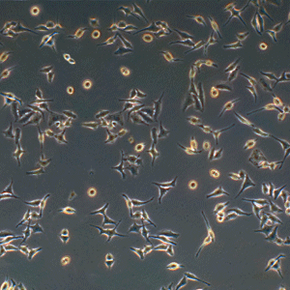
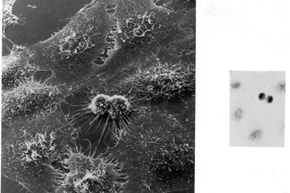
But HeLa cells were the first -- the first line of human cells to survive in vitro (in a test tube). Named after a cancer patient, the cells were taken from Lacks' tissue samples and grown by a researcher named Dr. George Gey in 1951. Dr. Gey quickly realized that some of Lacks' cells were different from normal cells. While those died, they just kept on growing. After more than 50 years, there are now billions and billions of HeLa cells in laboratories all over the world. It's the most commonly used cell line, and it's known to be extremely resilient.
The fact that HeLa cells have been used in some very important, groundbreaking medical research is interesting enough, but there's another part of the story -- and that part is why Oprah might be making a movie about HeLa. Henrietta Lacks had no idea that her cells were taken and used in this way, and neither did her family. And while the cells became commercialized (researchers can buy a vial of them for $250) Lacks' family has lived without healthcare and in poverty. Henrietta Lacks' story isn't just about her contribution to medical research; it's about the ethics of biomedical research and the practice of informed consent. But let's start at the beginning, with Henrietta herself.
Advertisement