Imagine for a moment that you have a movement disorder such as Parkinson's disease. The slight tremor that you first noticed in your fingertips has gradually worsened. Now simple tasks, like lifting a glass of water or even tying your shoes, have become nearly impossible. Your prescription medications were helpful for a time, but now the side effects are becoming a problem.
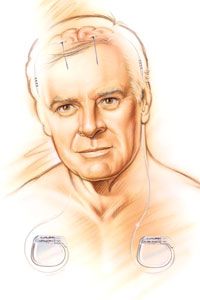
One day, your doctor suggests that you might be a good candidate for a relatively new therapy called deep brain stimulation. He describes how a small electrode would be implanted into a specific area of your brain, where it would deliver short pulses of electricity. These electrical pulses, he explains, would alter the patterns of activity in your brain responsible for your disease symptoms.
Advertisement
You decide to undergo the surgery required to implant the device, and just a few weeks later the difference is astonishing. Switching on the electrical stimulation immediately reduces your muscle tremors and restores your control over fine movements. Although your disease is still present, you can now manage its symptoms much more effectively.
This scenario is very real for the tens of thousands of people worldwide who have been implanted with a deep brain stimulation (DBS) device. In this article, we'll learn exactly how DBS works to achieve its therapeutic effects. We'll also explore what conditions can be treated with DBS and take a look at the risks and limitations of this form of treatment.
On the next page, we'll learn about the origins of deep brain stimulation and find out how the technology behind DBS was able to advance so quickly.
Advertisement