Condensation and Addition Reactions
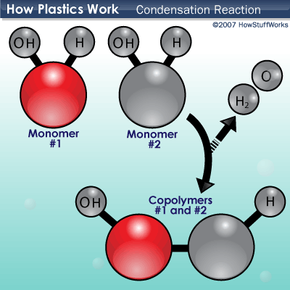
There are a few ways that monomers combine to form the polymers of plastics. One method is a type of chemical reaction called a condensation reaction. In a condensation reaction, two molecules combine with the loss of a smaller molecule, usually water, an alcohol or an acid. To understand condensation reactions, let's look at another hypothetical polymer reaction.
Monomers 1 and 2 both have hydrogen (H) and hydroxyl groups (OH) attached to them. When they come together with an appropriate catalyst (an atom or a molecule that speeds up the chemical reaction without being used up in it), one monomer loses a hydrogen while the other loses a hydroxyl group. The hydrogen and hydroxyl groups combine to form water (H2O), and the remaining electrons form a covalent chemical bond between the monomers. The resulting compound is the basic subunit of copolymers 1 and 2. This reaction occurs over and over again until you get a long chain of copolymers 1 and 2.
Advertisement
Another way that monomers can combine to form polymers is through addition reactions. Addition reactions involve rearranging electrons of the double bonds within a monomer to form single bonds with other molecules. Imagine that two people (each a monomer) stand close together and each person has his/her arms folded (double bond). Then they unfold their arms and hold hands (single bond). The two people now make a polymer, and the process can be repeated.
Various polymer chains can interact and cross-link by forming strong or weak bonds between monomers on different polymer chains. This interaction between polymer chains contributes to the properties of specific plastics (soft/hard, stretchy/rigid, clear/opaque, chemically inert).
Now we'll learn about the different types of plastic.