Wave Functions
The wave function of each electron can be described as a set of three quantum numbers:
- Principal number (n) - describes the energy level.
- Azimuthal number (l) - how fast the electron moves in its orbit (angular momentum); like how fast a CD spins (rpm). This is related to the shape of the orbital.
- Magnetic (m) - its orientation in space.
It was later suggested that no two electrons could be in the exact same state, so a fourth quantum number was added. This number was related to the direction that the electron spins while it is moving in its orbit (i.e., clockwise, counterclockwise). Only two electrons could share the same orbital, one spinning clockwise and the other spinning counterclockwise.
Advertisement
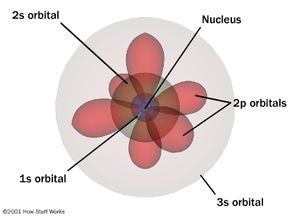
The orbitals had different shapes and maximum numbers at any level:
- s (sharp) - spherical (max = 1)
- p (principal) - dumb-bell shaped (max = 3)
- d (diffuse) - four-lobe-shaped (max = 5)
- f (fundamental) - six-lobe shaped (max = 7)
The names of the orbitals came from names of atomic spectral features before quantum mechanics was formally invented. Each orbital can hold only two electrons. Also, the orbitals have a specific order of filling, generally:
However, there is some overlap (any chemistry textbook has the details).
The resulting model of the atom is called the quantum model of the atom.
Sodium has 11 electrons distributed in the following energy levels:
- one s orbital - two electrons
- one s orbital - two electrons and three p orbitals (two electrons each)
- one s orbital - one electron
Right now, the quantum model is the most realistic vision of the overall structure of the atom. It explains much of what we know about chemistry and physics. Here are some examples:
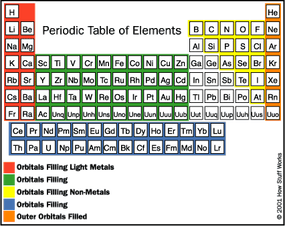
- Chemistry: The Periodic Table - the Table's pattern and arrangement reflects the arrangement of electrons in the atom. Elements have different atomic numbers - the number of protons or electrons increases up the table as electrons fill the shells. Elements have different atomic masses - the number of protons plus neutrons increases up the table. Rows - elements of each row have the same number of energy levels (shells). Columns - elements have the same number of electrons in the outermost energy level or shell (one to eight). Chemical reactions - exchange of electrons between various atoms (giving, taking, or sharing). Exchange involves electrons in the outermost energy level in attempts to fill the outermost shell (i.e., most stable form of the atom).
- Physics Radioactivity - changes in the nucleus (i.e., decay) emit radioactive particles. Nuclear reactors - splitting the nucleus (fission) Nuclear bombs - splitting the nucleus (fission) or forming a nucleus (fusion) Atomic spectra - caused by excited electrons changing energy levels (absorption or emission of energy in the form of light photons).