Quantum Mechanics: Putting It All Together
At the same time that discoveries were being made with radioactivity, physicists and chemists were studying how light interacted with matter. These studies began the field of quantum mechanics and helped solve the structure of the atom.
Quantum Mechanics Sheds Light on the Atom: The Bohr Model
Physicists and chemists studied the nature of the light that was given off when electric currents were passed through tubes containing gaseous elements (hydrogen, helium, neon) and when elements were heated (e.g., sodium, potassium, calcium, etc.) in a flame. They passed the light from these sources through a spectrometer (a device containing a narrow slit and a glass prism).
Advertisement

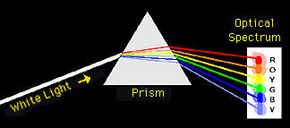
Now, when you pass sunlight through a prism, you get a continuous spectrum of colors like a rainbow. However, when light from these various sources was passed through a prism, they found a dark background with discrete lines.


Each element had a unique spectrum and the wavelength of each line within a spectrum had a specific energy (see How Light Works for details on the relationship between wavelength and energy).
In 1913, a Danish physicist named Niels Bohr put Rutherford's findings together with the observed spectra to come up with a new model of the atom in a real leap of intuition. Bohr suggested that the electrons orbiting an atom could only exist at certain energy levels (i.e., distances) from the nucleus, not at continuous levels as might be expected from Rutherford's model. When atoms in the gas tubes absorbed the energy from the electric current, the electrons became excited and jumped from low energy levels (close to the nucleus) to high energy levels (farther out from the nucleus). The excited electrons would fall back to their original levels and emit energy as light. Because there were specific differences between the energy levels, only specific wavelengths of light were seen in the spectrum (i.e., lines).
The major advantage of the Bohr model was that it worked. It explained several things:
Atomic spectra - discussed above
Periodic behavior of elements - elements with similar properties had similar atomic spectra.
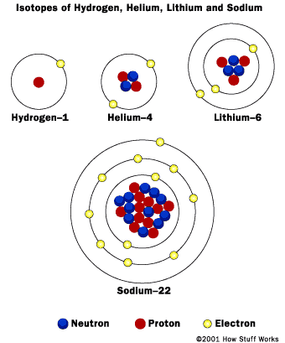
- Each electron orbit of the same size or energy (shell) could only hold so many electrons. For example, the first shell could hold two electrons, the second could hold eight electrons, the third could hold 18 electrons, the fourth 32 and so on until reaching the seventh.
- When one shell was filled, electrons were found at higher levels.
- Chemical properties were based on the number of electrons in the outermost shell. Elements with full outer shells do not react. Other elements take or give up electrons to get a full outer shell.
As it turns out, Bohr's model is also useful for explaining the behavior of lasers, although these devices were not invented until the middle of the 20th century.
Bohr's model was the predominant model until new discoveries in quantum mechanics were made.