Can We See Atoms?
Atoms are so small that we cannot see them with our eyes (i.e., microscopic). To give you a feel for some sizes, these are approximate diameters of various atoms and particles:
- atom = 1 x 10-10 meters
- nucleus = 1 x 10-15 to 1 x 10 -14 meters
- neutron or proton = 1 x 10-15 meters
- electron - not known exactly, but thought to be on the order of 1 x 10-18 meters
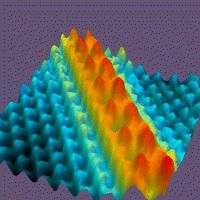
You cannot see an atom with a light microscope. However, in 1981, a type of microscope called a scanning tunneling microscope (STM) was developed. The STM consists of the following:
Advertisement
- A very small, sharp tip that conducts electricity (probe)
- A rapid piezoelectric scanning device to which the tip is mounted
- Electronic components to supply current to the tip, control the scanner and accept the signals from the motion sensor
- Computer to control the system and do data analysis (data collection, processing, display)
The STM works like this:
- A current is supplied to the tip (probe) while the scanner rapidly moves the tip across the surface of a conducting sample.
- When the tip encounters an atom, the flow of electrons between the atom and the tip changes.
- The computer registers the change in current with the x,y-position of the atom.
- The scanner continues to position the tip over each x,y-point on the sample surface, registering a current for each point.
- The computer collects the data and plots a map of current over the surface that corresponds to a map of the atomic positions.
The process is much like an old phonograph where the needle is the tip and the grooves in the vinyl record are the atoms. The STM tip moves over the atomic contour of the surface, using tunneling current as a sensitive detector of atomic position.
The STM and new variations of this microscope allow us to see atoms. In addition, the STM can be used to manipulate atoms as shown here:

Atoms can be moved and molded to make various devices such as molecular motors (see How Nanotechnology Will Work for details).
In summary, science in the 20th century has revealed the structure of the atom. Scientists are now conducting experiments to reveal details of the structure of the nucleus and the forces that hold it together.
Related HowStuffWorks Articles
More Great Links
- The Atoms Family: The Phantom's Portrait Parlor - activities about atoms for grades 6-12
- American Institute of Physics Exhibit: A Look Inside the Atom
- All About Atoms
- Anatomy of the Atom
- Journey into the Atom - learn about the atom and particle physics
- American Institute of Physics Exhibit: The Discovery of the Electron
- Physical Structure: Atomic Structure
- Physical Structure: Main Menu
Chemistry
Periodic Table
Orbitals
- Orbitals Tutorial - media shockwave animation
Atomic Models
- Physics 2000: Quantum Atom Tutorial - excellent Java applet demonstrations; use table of contents to see elements as atoms and Periodic Table as well