A Closer Look at Laser-induced Breakdown Spectroscopy
Laser-induced breakdown spectroscopy, or LIBS, has been advancing significantly over the last decade. It can analyze solids, liquids and gases and can return results rapidly, with very little damage to the sample. Not only that, it can do its work from a distance, unlike some analytical tools that require samples being brought to a lab. For example, LIBS is being used to detect surface contaminants in a few nuclear reactors around the world. The laser in these systems is located several meters from the reactor surface and yet is still able to function effectively. These systems keep most of the instrumentation behind shielding material, with only a mirror and a lens (which are used to steer and focus the laser beam respectively) exposed to the nuclear radiation.
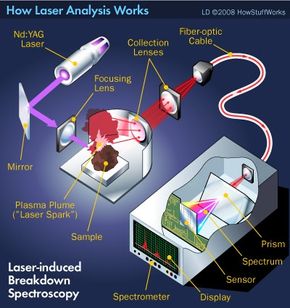
We'll consider other practical applications of LIBS in a moment, but how exactly does it work? Like LA-ICP-OES, LIBS uses a laser to cut tiny particles from the surface of a sample. But in LIBS, the laser itself creates the plasma instead of a plasma torch. Let's take a look at the four major parts of a typical LIBS system and how they work. The diagram above shows a schematic of the setup.
Advertisement
- The laser, of course, is the business end of the instrument. Generally, LIBS systems use a neodymium-doped yttrium aluminum garnet (Nd:YAG) laser at its fundamental wavelength of 1,064 nanometers, but many different lasers have been used. The laser doesn't blast the sample with a nonstop beam. Instead, it shoots pulses, with each pulse lasting about 5 to 20 nanoseconds.
- The laser light passes through a lens, which focuses the energy onto the sample. Some systems work on the laboratory bench and accommodate small samples, maybe a few centimeters thick, placed inside a chamber. Other systems can be carried to a remote site and used to analyze larger objects. In either case, the more tightly focused the laser, the less energy is required to break down the sample. In fact, the laser pulses in LIBS typically carry energies of only 10 to 100 millijoules. To put that into context, think of the energy required to lift an apple one meter straight up. That's equivalent to a joule. One millijoule is 0.001 joules -- considerably less energy. And yet that's still enough to ablate some of the sample material. As the particles are removed from the sample surface, they are ionized to form a small plume of plasma, what chemists call a "laser spark."
- As the plasma plume expands, constituent atoms in the ionized gas become excited. Over just a few microseconds, the excited atoms began to relax, resulting in characteristic spectral emissions. The emitted light travels through a series of collecting lenses, which focus the light and deliver it to a fiber-optic system. The fiber-optic system carries the light to a spectrometer.
LIBS has several benefits. Because the sample requires no special preparation, the process is relatively simple and inexpensive. Not only that, LIBS can be used to determine the elemental composition of any sample, unlike certain techniques that are great at analyzing solids, but not liquids and gases. Even very hard materials are fair game because the lasers carry so much energy. But one of the greatest benefits of LIBS is its ability to provide information without destroying the sample. The laser removes less than a milligram of material, which is practically invisible. As we'll see on the next page, this makes LIBS an ideal solution for analyzing valuable items, such as paintings or archaeological artifacts.