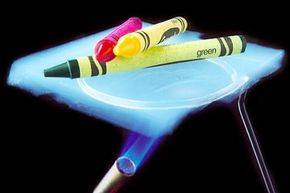
The three most common types of aerogels are silica, carbon and metal oxides, but it's silica that is most often used experimentally and in practical applications. When people talk about aerogels, chances are they're talking about the silica type [source: Aerogel.org, Silica]. Silica is not to be confused with silicon, which is a semiconductor used in microchips. Silica is a glassy material often used for insulation.
Carbon Aerogel Insulation Material
Unlike the smoky-blue silica aerogels, carbon-based ones are black and feel like charcoal to the touch. What they lack in looks, they make up for in high surface area and electrically conductive capabilities. These properties make carbon aerogels useful for supercapacitors, fuel cells, and desalination systems [source: Aerogel.org, Organic].
Metal Aerogel Insulation Material
Metal oxide aerogels are made from metal oxides and are used as catalysts for chemical transformations. They are also used in the production of explosives and carbon nanotubes, and these aerogels can even be magnetic. What sets metal oxide aerogels such as iron oxide and chromia apart from their more common silica cousins is their range of startlingly bright colors. When made into an aerogel, iron oxide lends an aerogel in its trademark rust color. Chromia aerogels appear deep green or blue. Each type of metal oxide results in an aerogel of a slightly different color. [source: Aerogel.org, Metal].
Silica Aerogel Insulation Material
The most common type, silica aerogels are blue for the same reason the sky is blue. The blue color occurs when white light encounters the aerogel's silica molecules, which are larger than the wavelengths of light. The aerogel scatters, or reflects, the shorter wavelengths of light more easily than the longer ones. Because blue and violet light have the shortest wavelengths, they scatter more than other colors of the visible spectrum. We see scattered wavelengths as color, and since our eyes are more sensitive to blue wavelengths, we never see the violet ones [source: Steiner, Zero-Gravity].
Read on to learn more about aerogels' applications in space.