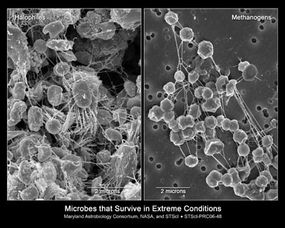
An environment is called extreme only in relation to what's normal for humans, but for an extremophile, their favored environments are "normal." And beyond Earth, conditions that make life possible for humans are likely rare. In turn, so-called extreme environments and the extremophiles that populate them may be more commonplace.
Here on Earth, a number of factors might earn a place the label "extreme," including the following:
- Pressure
- Radiation levels
- Acidity
- Temperature
- Salinity
- Lack of water
- Lack of oxygen
- Pollutants or toxins left behind by humans (oil, nuclear waste, heavy metals)
Remember that these factors can sometimes be extreme in one of two ways: very hot or very cold, highly acidic or highly alkaline. Most organisms that we see or encounter subsist in temperatures ranging from 41 degrees Fahrenheit (5 degrees Celsius) to 104 degrees Fahrenheit (40 degrees Celsius), but extreme life has been found in nuclear reactors, penguin guano, volcanoes, practically oxygen-free zones, incredibly salty areas like Utah's Great Salt Lake and in the digestive systems of many animals, including insects [source: Science Education Resource Center].
In one case of thriving in extreme conditions, bacteria were found entombed in Alaskan ice. When the ice melted, bacteria that had been dormant for tens of thousands of years resumed activity, as if nothing had happened.
Antarctica's Lake Untersee is a great example of an extreme environment. The water is brimming with methane and has a highly alkaline pH, comparable to laundry detergent [source: Science Daily]. Scientists at NASA are particularly interested in the lake because its distinct environment — lots of methane and low temperatures — may be similar to those of other planetary bodies, such as Jupiter's moon Europa [source: NASA].
Acidic Environments
As we've mentioned, some of these organisms love a good acidic environment. But just how acidic? First, let's take a look at how acidity is measured. "Potential of hydrogen," or pH, is a measure of the acidity or alkalinity of a substance, indicating the concentration of hydrogen ions (H+) in a solution. In terms of pH: 0 is most acidic, while 14 is most basic or alkaline.
Humans prefer a pH of 6.5 to 7.5, but acidophiles thrive in places with pH levels ranging from 0 to 5. The human stomach actually falls into this category, and we have some extremophiles living in our bodies.
In general, acidophiles survive in acidic environments by strengthening their cell membranes. Some produce biofilms (colonies of microorganisms that aggregate, creating slimy, extracellular protective films) or fatty acids that protect their cell membranes. Others can regulate their internal pH to keep it at a more moderate level of around 6.5.
Extremophiles in highly alkaline environments also manage to regulate internal pH and have enzymes that can withstand the effects of high alkalinity.
One such extremophile is Spirochaeta americana, a bacteria that lives in the mud deposits of California's Mono Lake and whose discovery was announced in May 2003. One of 14 known spirochetes, this extremophile needs an alkaline pH from 8.0 to 10.5. And it's anaerobic, which means it's incapable of living in environments with oxygen.
Spirochetes like sulfurous mud deposits and don't rely on oxygen. For example, Spirochaeta thermophila lives near deep-sea hydrothermal vents. Mono Lake's mud is alkaline with a pH of 10, very salty and filled with sulfides. The lake became this way because it's a terminal lake — water flows in but not out. As water evaporates, chemicals and minerals stay, becoming highly concentrated.
Geysers and Rivers
Many other notable extreme environments also play host to extremophiles. Numerous geysers around the world, including some in Siberia, have extremophiles living in their hot pools and vents.
In the United States, Yellowstone National Park has thousands of geysers, springs and other geothermal features, with varying levels of temperature, acidity and sulfur — and many types of extremophiles.
Rio Tinto, a river in Spain, is full of heavy metals because the region has been host to mining operations for thousands of years. Similarly, Northern California's Iron Mountain has water so loaded with heavy metals and acids (byproducts of mining) that it can eat through a metal shovel in a day.
But even here, deep in underground mines, microbes from the Archaea and Eubacteria domains manage to survive scrappily, using biofilms for both protection and nutrient absorption.