Key Takeaways
- Acid rain, with a pH of 5.0 or less, is formed when pollutants like sulfur dioxide and nitrogen oxides from burning fossil fuels react with water vapor in the atmosphere, creating sulfuric and nitric acids.
- Acid rain can damage aquatic ecosystems by lowering pH levels and killing sensitive species, weakening trees and contributing to nitrogen deposition that leads to harmful algae blooms.
- Efforts to monitor and reduce the emissions that cause acid rain include national programs like the National Atmospheric Deposition Program (NADP) and the Clean Air Status and Trends Network (CASTNET).
If you hike through the Appalachian Mountains, you'll spot stands of dead and weakened trees. If you live in a city, you might notice worn stone buildings, streaks on your car roof or corroded metal railings and statues. You can see the effects of acid rain nearly everywhere you go, but with media and public attention turned to the more ominous prospect of global warming, acid rain has fallen by the wayside. The scourge from the sky almost seems like a 20th-century problem -- an issue dealt with in the 1980s and 1990s by legislation.
Acid rain occurs mostly in the Northern Hemisphere -- the more industrialized, dirtier half of the globe. Winds can sweep up emissions from high smokestacks and carry pollutants far from their original sources, crossing state lines and national borders in the process. Acid rain may not have the complete global range of greenhouse gases, but it is a transboundary, and therefore international, issue.
Advertisement
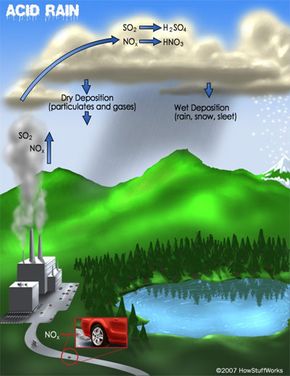
Acid rain, also known as acid deposition, is caused by emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) from power plants, cars and factories. Natural sources like volcanoes, forest fires and lightning strikes also add to the man-made pollution. SO2 and NOx become acids when they enter the atmosphere and react with water vapor. The resulting sulfuric and nitric acids can fall as wet or dry depositions. Wet deposition is precipitation: acid rain, snow, sleet or fog. Dry deposition falls as acidic particulates or gases.
Advertisement