Only a sliver of the world's water is fresh to begin with, and after subtracting gluttons like glaciers and ice caps, all that's left is a drop in the proverbial bucket. Since humans and a fair portion of the planet's plant and animal population can't subsist on saltwater, people have long looked enviously at the sea to provide the water they require, whether it's for drinking, hygiene, agriculture or more recently, industrial purposes.
Historically, desalination was deemed too expensive to be considered a viable large-scale option; it simply required too much energy. But newer technologies, such as reverse osmosis and multistage flash distillation, have, since the 1950s, slowly started to change that opinion, especially in places where sources of freshwater are scarce, and people are plentiful. To read more about the actual processes at work, check out How Desalination Works. But for this article, let's take a closer look at how desalination operates in the real world.
Advertisement
Take Australia, for example. At the Water Services Association of Australia, employees consider their arid continent a forecast for what may be the future of water supply systems in an increasingly hot and dry world. In the midst of an extended drought, the country's five largest cities began preparing for water shortages by building massive desalination plants for $13.2 billion [source: New York Times]. The plants certainly have their critics -- citizens complaining about higher water bills, conservationists arguing against the environmental effects of the plants, and economists saying other options would have been more fiscally responsible. But according to regional water authorities, the area is now set to handle drought and water supply issues well into the future.
Israel is another instance of desalination in action. Among the many nations in the Middle East attempting to ward off water shortages, Israel is counting on desalination plants. The third of five major plants planned off the coast of Israel went into operation in January 2010, and, for now, it's the largest reverse osmosis desalination plant on Earth. Once all the facilities are complete, they're expected to provide about two thirds of the country's drinking water [source: Associated Press].
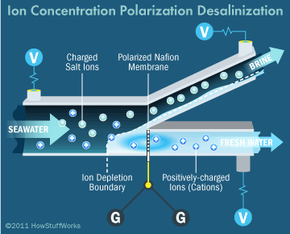
Desalination techniques are also being developed on a much smaller scale. Portable desalination kits are a prime example. Researchers at MIT are working to bring desalination down to the nano level, harnessing electrostatic ion-selective membranes to avoid requirements and disadvantages often associated with the reverse osmosis method, like the need for high levels of pressure and the occurrence of inadvertent clogs and foulings. They call the process ion concentration polarization, and they envision it helping in disaster zones. The units wouldn't produce the vast amounts of freshwater rendered by plants, but they would be self-contained, portable and powered by solar cells or batteries. Many units could be distributed during relief efforts and provide drinkable water until infrastructure functionality was reestablished [source: MIT News].
It seems the future of desalination is wide open with different research institutions continually looking for ways to make the process more efficient and more cost effective. Sooner or later, we might enjoy the occasional long, cool sip of water that, left untreated, would have been a deadly cocktail.
Advertisement