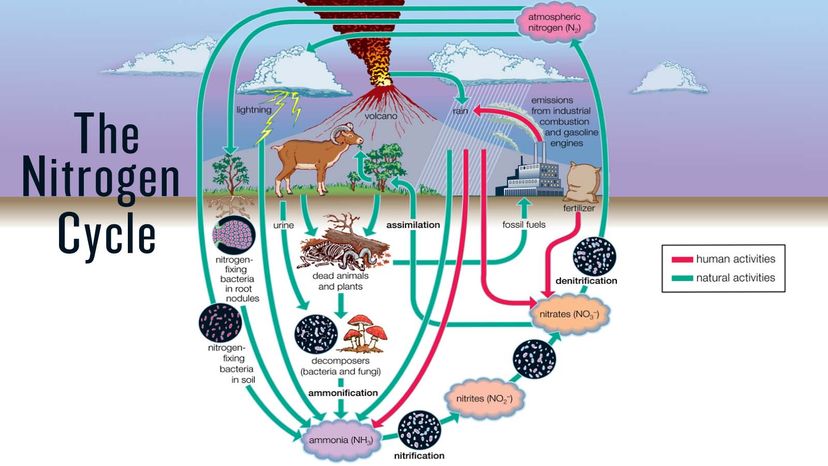
Living things require nitrogen for their cells to function and, furthermore, we are virtually steeping in the stuff since our atmosphere is made up of 78 percent nitrogen gas.
Although nitrogen's lurking basically everywhere, it's not abundant in the Earth's crust, and it's difficult for living things to capture atmospheric nitrogen and use it for their purposes. The nitrogen cycle steps are kind of like a currency exchange, converting nitrogen into different forms, some of which plants and animals can use, and some of which they can’t.
Advertisement
"Nitrogen is a major part of amino acids, which are the building blocks of proteins and nucleic acids such as DNA," says Jessie Motes, a Ph.D. candidate in the Odum School of Ecology at the University of Georgia, in an email. "In addition to needing nitrogen for proteins in plants, it is a main component of chlorophyll, which makes it crucial for photosynthesis."
