As you may already know, everything is made of atoms, which bind together into molecules. For instance, a water molecule is made from two hydrogen atoms and one oxygen atom bound together into a single unit.
In nature, any atom you find will be one of 92 types of atoms, also known as elements. So every substance on Earth — metal, plastics, hair, clothing, leaves, glass — is made up of combinations of the 92 atoms found in nature. The Periodic Table of Elements you see in chemistry class is a list of the elements found in nature, plus a number of man-made elements.
Negative, Positive and Neutral
Inside every atom are three subatomic particles: protons, neutrons and electrons. Protons and neutrons bind together to form the nucleus of the atom, while the electrons surround and orbit the nucleus.
Protons and electrons have opposite charges and therefore attract one another (electrons are negative and protons are positive, and opposite charges attract). In most cases, the number of electrons and protons are the same for an atom (making the atom neutral in charge).
The neutrons are neutral. Their purpose in the nucleus is to bind protons together. Because the protons all have the same charge and would naturally repel one another, the neutrons act as "glue" to hold the protons tightly together in the nucleus.
Behave, Atom
The number of protons in the nucleus determines the behavior of an atom. For example, if you combine 13 protons with 14 neutrons to create a nucleus and then spin 13 electrons around that nucleus, what you have is an aluminum atom. If you group millions of aluminum atoms together you get a substance that is aluminum — you can form aluminum cans, aluminum foil and aluminum siding out of it.
All aluminum that you find in nature is called aluminum-27. The "27" is the atomic mass number, or the sum of the number of neutrons and protons in the nucleus. If you take an atom of aluminum and put it in a bottle and come back in several million years, it will still be an atom of aluminum. Aluminum-27 is therefore called a stable atom.
Up to about 100 years ago, scientists thought that all atoms were stable like this, but many atoms come in different forms.
For example, copper has two stable forms: copper-63 (making up about 70 percent of all natural copper) and copper-65 (making up about 30 percent). The two forms are called isotopes. Atoms of both isotopes of copper have 29 protons, but a copper-63 atom has 34 neutrons while a copper-65 atom has 36 neutrons. Both isotopes act and look the same, and both are stable.
Radioactive Material
The part that scientists didn't understand until about 100 years ago is that certain elements have isotopes that are radioactive.
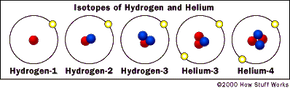
In some elements, all of the isotopes are radioactive. Hydrogen is a good example of an element with multiple isotopes, one of which is radioactive. Normal hydrogen, or hydrogen-1, has one proton and no neutrons. (Because there is only one proton in the nucleus, there is no need for the binding effects of neutrons.)
Another isotope, hydrogen-2 (also known as deuterium), has one proton and one neutron. Deuterium is very rare in nature, making up about 0.015 percent of all hydrogen. While it acts like hydrogen-1 (for example, you can make water out of it), it can be toxic in high concentrations. The deuterium isotope of hydrogen is stable.
A third isotope, hydrogen-3 (also known as tritium), has one proton and two neutrons. It turns out this isotope is unstable. That is, if you have a container full of tritium and come back in a million years, it will have turned into helium-3 (two protons, one neutron), which is stable. The process by which it turns into helium is called radioactive decay.
Certain elements are naturally radioactive in all of their isotopes. Uranium is the best example of such an element and is the heaviest naturally occurring radioactive element. There are eight other naturally radioactive elements: polonium, astatine, radon, francium, radium, actinium, thorium and protactinium. All other man-made elements heavier than uranium are radioactive as well.