Two explorers, searching the depths of a giant cave, collect various samples of rocks and minerals for research. They've descended into an area never before touched by human hands nor seen by human eyes, so they must be extra careful not to disturb the natural formations. One false step could upset thousands of years of peace and quiet. But as one explorer absent-mindedly admires the shimmering beauty of the cave, the other urgently calls out: "Watch out for that stalagmite!" The explorer looks up, but he's unfortunately made a horrible mistake -- he's mixed up stalactites and stalagmites, and a second later he steps on a precious stalagmite and breaks it.
It's one of those timeless questions that plague us from elementary school on, right up there with "Why is the sky blue?" What exactly is the difference between stalactites and stalagmites? Which one hangs above and which one stands up from the ground?
Advertisement
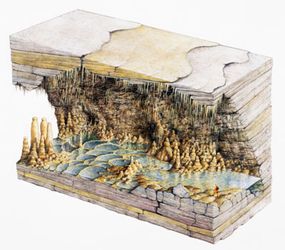
Stalactites and stalagmites are what are known as speleothems, deposits of minerals that form into cave structures and line the insides of a cave. Stalactites are the formations that hang from the ceilings of caves like icicles, while stalagmites look like they're emerging from the ground and stand up like a traffic cone. Some may take thousands of years to form, while others can grow quite rapidly. The two formations are also sometimes referred to collectively as dripstone.
Is that all there is to stalactites and stalagmites, or are there any more differences between the two formations? How is each one formed, for instance? Do they form independently from each other or at the same time? To learn about speleothems, read about famous cave formations and find out some of the classic memory tricks to remember the difference between stalactites and stalagmites, see the next page.
Advertisement