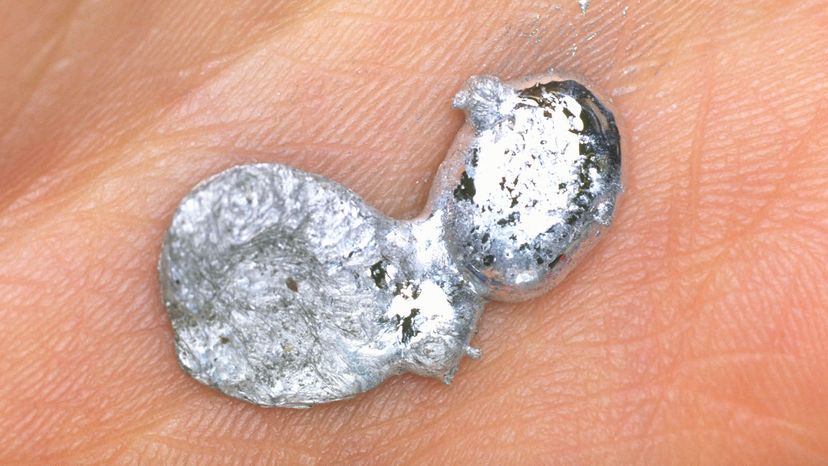
Gallium is a rare, silvery white element that can pull off one of the coolest parlor tricks on the periodic table. At room temperature, gallium is a shiny metallic solid that resembles pure aluminum. But hold it in your hands for a few minutes and this solid hunk of metal starts to melt.
Yup, the melting point of gallium is just 85.6 degrees F (29.8 degrees C), which means that it melts into a mirror-like puddle in your hot little hand. In its liquid form, gallium looks a lot like mercury, but gallium isn't toxic like mercury so it's safer to handle (although it can stain your skin).
Advertisement
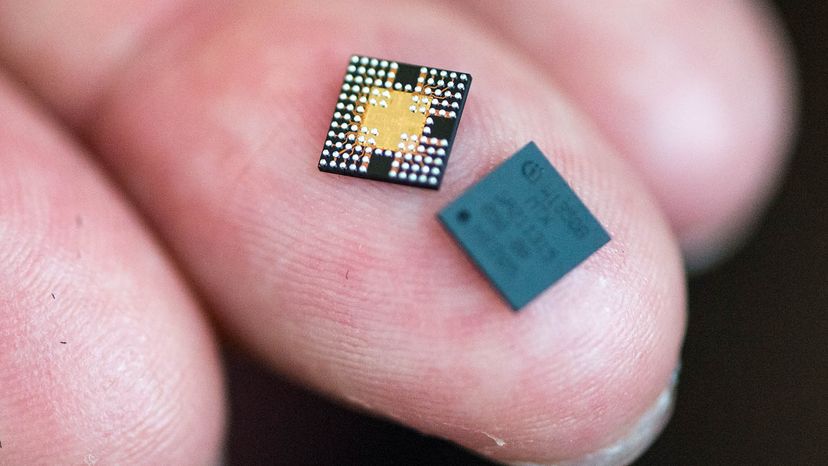
But gallium is so much more than fodder for melt-in-your-hand YouTube videos. It's also a key ingredient in LED lights and the go-to semiconductor material for the powerful microchips in your smartphone. The only thing stopping gallium from taking over the electronics world is that it's very rare and very expensive compared to silicon.