The Science of a Scandal: Real-world Mass Spectrometry
Let's get back to the baseball player's urine we discussed in the introduction. In mass spectrometry, the urine would be called a sample. Once upon a time, mass spectrometers were only capable of analyzing samples that existed as gases, but today's models can handle solids and liquids. Spectrometrists, usually analytical chemists, insert the sample directly into the ionization chamber or, if it's a complex mixture, into another device that performs a first-pass separation of the sample's component parts. Chromatography is the most common way to conduct this initial separation and can occur as either gas chromatography (GC) or liquid chromatography (LC). Chromatography separates the sample into a series of components by first dissolving the substance in a gas or a liquid, then forcing it through a secondary material. A component that is soluble in the first phase will move more slowly than a component that is not soluble in the first phase but very soluble in the second phase. As a result, the various components become separated. Each one then enters the mass spectrometer for analysis.
Urine drug testing is typically conducted by gas chromatography/mass spectrometry (GC/MS). Sometimes, more than one mass spectrometer is used in a technique called tandem mass spectrometry, which basically acts to break apart large ions into smaller ions for more detailed analysis. All of this is required because urine contains a large number of components, including naturally occurring steroids. Using GC/MS or GC/MS/MS detects more chemicals and yields more reliable results.
Advertisement
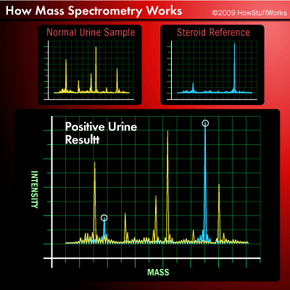
How do chemists use mass spectrometry to test for illegal steroids? First, they analyze several known steroids to produce their mass spectra for comparison purposes. They can also rely on tables of mass values, if they are available for reference. Next, they analyze normal, uncontaminated urine. Then they test a player's urine, using either GC/MS or GC/MS/MS. Finally they compare the mass spectrum produced from the player's urine with the spectra of normal urine and known steroids. By comparing the peaks on the spectra, which represent ions of various masses, chemists can identify exactly what drugs, if any, are present in the urine. Typical results might look those shown below.
Notice that the test sample has peaks that line up with the steroid standard. The peaks represent ions containing certain elements. For example, the C2H3 ion will have a different peak than the OH ion. In this way, mass spectra are used as "fingerprints" to identify compounds. Up next, we'll see that those compounds don't have to be in a ball player's urine. They could be in almost any sample imaginable.