Building the Periodic Table Block by Block
Each block of the periodic table houses an element, along with a few standard facts about that element:
- Atomic number: integer equal to the number of protons or electrons in the element. Gold's atomic number is 79.
- Element symbol: one or two letters. In the case of two letters, the first one is always capitalized. Hydrogen's symbol is just H, while helium's is He. Symbols can be tricky because some are based on the first letter(s) of the element's common name, as hydrogen's is, while other symbols are based on the Latin names of the element, such as Au for gold (or aurum in Latin).
- Element name
- Atomic weight: usually a decimal value, such as 196.966 569(4) for gold
Some periodic tables include the electron configuration (arrangement of electrons) in a corner of the block or below the name of the element. In addition, some periodic tables thoughtfully include colored symbols to indicate whether the element is a solid, liquid or gas at standard temperature (25 degrees C or 77 degrees F) and colored backgrounds to indicate the type of element (alkali metals, alkaline earth metals, nonmetals, noble gases and so on).
Advertisement
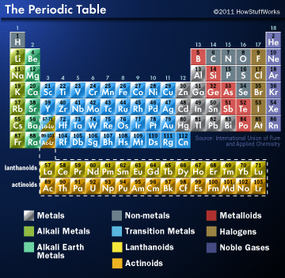
Within the table, the elements are arranged by increasing atomic number, as you'll recall. The elements stretch across seven rows. Each row is called a period and indicates the energy levels or shells occupied by the electrons around the nucleus of that element (see How Atoms Work). For example, the first energy level can only hold two electrons max, so hydrogen and helium occupy period 1. In period 2, the second energy level begins to fill. The pattern continues. The elements in period 7 have enough electrons to start filling the seventh energy level. No known element yet has eight energy levels.
Each energy level above the first one has sublevels or orbitals. The orbitals are s (sharp), p (principal), d (diffuse) and f (fundamental). But electrons don't fill directly in the order of s then p then d then f. That would be too easy. There's some overlap between the orbitals of one energy level and those of the one below it. For example, electrons in the fourth energy level fill in this order: 4s then 3d then 4p. (If you can't quite picture it, the American Chemical Society has a periodic table that allows you to see how the various electron configurations work here.)
As the atomic number increases and one energy level fills, a new period begins. If you placed all of the elements in order of increasing atomic number, the periodic table would span more than one tidy sheet of standard paper. That's why chemist Glenn Seaborg suggested pulling out the lanthanoids and actinoids and placing them below the table to make it more compact.
The electrons of the outermost energy levels are the restless ones that participate in chemical reactions. So as each new period begins, there are elements that have similar chemical properties -- those with one outer electron, those with two, three and so on. Mendeleyev couldn't have predicted this periodic nature because he didn't know about atomic structure. But what about the columns?
