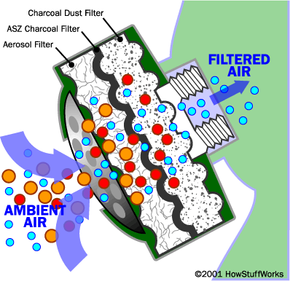
How Filters Work
Because of the problems with SCBA systems, any respirator that you are likely to use will have a filter that purifies the air you breathe. How does the filter remove poisonous chemicals and deadly bacteria from the air?
Any air filter can use one (or more) of three different techniques to purify air:
Advertisement
- Particle filtration
- Chemical absorption or adsorption
- Chemical reaction to neutralize a chemical
Particle filtration is the simplest of the three. If you have ever held a cloth or handkerchief over your mouth to keep dust out of your lungs, you have created an improvised particulate filter. In a gas mask designed to guard against a biological threat, a very fine particulate filter is useful. An anthrax bacteria or spore might have a minimum size of one micron. Most biological particulate filters remove particle sizes as small as 0.3 microns. Any particulate filter eventually clogs, so you have to replace it as breathing becomes difficult.
A chemical threat needs a different approach, because chemicals come as mists or vapors that are largely immune to particulate filtration. The most common approach with any organic chemical (whether it be paint fumes or a nerve toxin like Sarin) is activated charcoal.
Charcoal is carbon. (See this Question of the Day for details on how charcoal is made.) Activated charcoal is charcoal that has been treated with oxygen to open up millions of tiny pores between the carbon atoms. According to Encyclopedia Britannica:
The word adsorb is important here. When a material adsorbs something, it attaches to it by chemical attraction. The huge surface area of activated charcoal gives it countless bonding sites. When certain chemicals pass next to the carbon surface, they attach to the surface and are trapped.
Activated charcoal is good at trapping carbon-based impurities ("organic" chemicals), as well as things like chlorine. Many other chemicals are not attracted to carbon at all -- sodium and nitrates, to name a couple -- so they pass right through. This means that an activated-charcoal filter will remove certain impurities while ignoring others. It also means that, once all of the bonding sites are filled, an activated charcoal filter stops working. At that point you must replace the filter.
Sometimes, the activated charcoal can be treated with other chemicals to improve its adsorption abilities for a specific toxin.
The third technique involves chemical reactions. For example, during chlorine gas attacks in World War I, armies used masks containing chemicals designed to react with and neutralize the chlorine.
In industrial respirators, you can choose from a variety of filters depending on the chemical that you need to eliminate. The different filters are color coded by NIOSH standards for things like acids and ammonia. See this page for details.
Related HowStuffWorks Links
Other Great Links
Gas Masks
- Respirators (in particular the Millenium Chemical-Biological Mask)
- Civilian Gas Mask.com
- Quick Mask
- Respirator Selection
- NIOSH Pocket Guide to Chemical Hazards
Protective Clothing
- Chemical Defense Equipment
- Protection from Chemical and Biological Warfare
- Protection against terror weapons
- Chemical Protective Equipment Components
- Chemical and Biological Warfare: Are the United States Navy and Marine Corps prepared?