Chemical Elements
Chemical elements are substances composed of only one type of atom and they cannot be broken down further. Chemical elements are the simplest forms of matter and each one is assigned a specific atomic number. Check out these articles on chemical elements.
Learn More
Precious metals are not just shiny and attractive; these elements are also incredibly valuable, often used in a wide range of industries from jewelry to high-tech applications.
By Mack Hayden
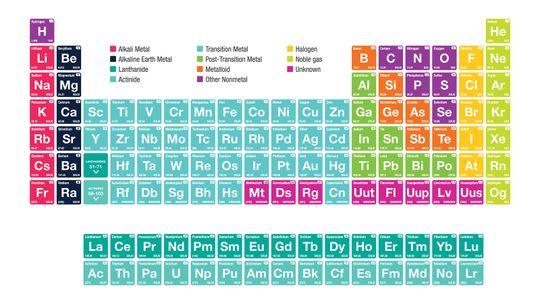
It's the ultimate cheat sheet for science class - and it's right there hanging on the wall. What do you really know about the indispensable periodic table of elements?
The air we breathe contains 21 percent oxygen. Would we be better off breathing 100 percent oxygen?
Advertisement
We love it. We wear it glittering around our necks and sparkling at our ears, wrists and feet. We pass it down to our children and hoard it in secret stashes. Why is this precious metal so prized?
It begins with an unassuming "H" and ends in crazy elements that you've likely never heard of. But the periodic table, encapsulated on a mere sheet of paper, can be a scientist's best friend and a testament to our human drive to organize the world.
Once considered a semiprecious metal alongside gold and silver, aluminum pretty much languished in obscurity until the 19th century. How did the metal become so ubiquitous?
Helium is the second lightest element on the Periodic Table. How is helium created?
Advertisement
Scientists at the Lawrence Berkeley National Laboratory just made history with einsteinium. They held a sample of the short-lived element long enough to measure some of its chemical properties.
By Dave Roos
Bismuth is a naturally occurring element with many applications in our daily lives, but even more than that, it looks amazing when it cools!
Diatomic elements are molecules composed of only two atoms, every time, always. There are only seven of them on the entire periodic table.
Don't know your fool's gold from the real deal? We'll tell you how to tell what's pyrite (aka fool's gold) and the good ol' 24 karat stuff you want.
By Mark Mancini
Advertisement
Tungsten's hardness and heat resistance make it a must for products like rocket engine nozzles, armor-piercing bullets and even the humble light bulb filament. In fact, pure tungsten boils at 10,030 F, the same as the photosphere of the sun.
By Dave Roos
Although the term might be unfamiliar, you know all about alkali metals. Ever used salt or eaten a banana? So, what special properties do these elements have?
More than fodder for melt-in-your-hand YouTube videos, gallium is a key component in LED lights and the powerful microchips in your smartphone.
By Dave Roos
Award-winning poet and fiction writer Mary Soon Lee has found a charming way to combine science and poetry in a refreshing new take on the periodic table of elements.
By Carrie Tatro
Advertisement
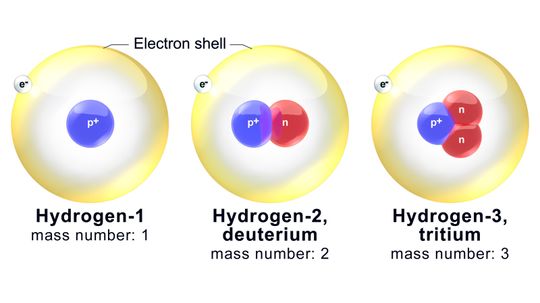
You don't need to be a fan of chemistry to appreciate isotopes. They affect geology and medicine, too.
By Mark Mancini
Discovered in the early 1800s from a chunk of smuggled platinum ore, rhodium is the most valuable precious metal on the planet today, used mainly for keeping car emissions in check.
Cadmium is a natural metal and the leading component in rechargeable batteries and solar cells. It is also highly toxic and heavily regulated.