Biological Fields
Biological fields are the different areas of study related to biology, such as botany, genetics and conservation. The various biological fields differ greatly in size, scope and methodology but all relate to the study of life.
Learn More
The natural world is a finely-tuned balance of biotic (living) and abiotic (nonliving) components that shape our environments. Various biotic factors directly affect processes like population growth, plant growth and nutrient cycling.
By Ada Tseng
Have you ever wondered what the largest living organism on Earth is? Well, you might be surprised to learn that it's not a giant blue whale or a sequoia tree; it's a fungus!
By Mack Hayden
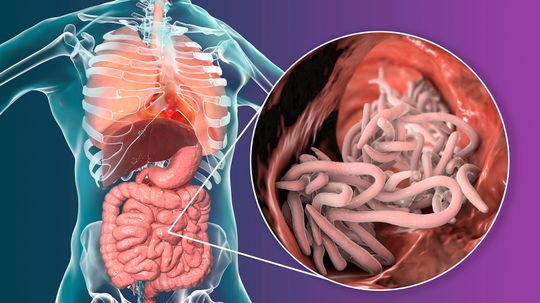
We're about to dive into the world of parasitology, taking a close look at one of the most common parasitic worms infecting humans: Ascaris lumbricoides. This large roundworm is responsible for a type of intestinal nematode infection that affects millions of people worldwide, especially in areas with poor sanitation.
By Mack Hayden
Advertisement
Jack Black does it. Wyclef Jean does it. And chances are, you do it, too. Everyone's a rock star in the bathroom. And there's a scientific explanation behind our soapy musical stylings.
By Debra Ronca
From tobacco smoke enemas to whirling chairs, doctors have tried almost everything to cure human disease.
A funny thing happens when you live in complete darkness. You lose your eyesight. At least that's what's happened to the species that have evolved inside our deepest, darkest caves.
By Debra Ronca
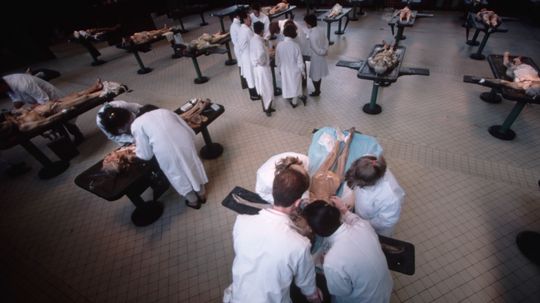
There's a great need for people to donate their bodies to science but not many people think about doing it. What happens to your body after you make that decision?
Advertisement
There are many myths and stigmas associated with leprosy, almost all completely incorrect. It's not a very contagious disease, and it's easily treatable. What else is wrong in the common beliefs about Hansen's disease?
They might look like piles of goop, but slime molds can think and seemingly make decisions without a brain.
How do we consider a Thing with no edge? Ecosystem ecologists are always trying.
Commensalism is a form of cooperation among species in which one species benefits from another without the first one suffering any harm from the relationship.
Advertisement
What are the chances there are still large, undiscovered animals on the planet? More likely than you might think.
By Diana Brown
On the surface, Antarctica may seem like a barren landscape. But underneath, in massive ice caves, life may be abundant.
By Amanda Onion
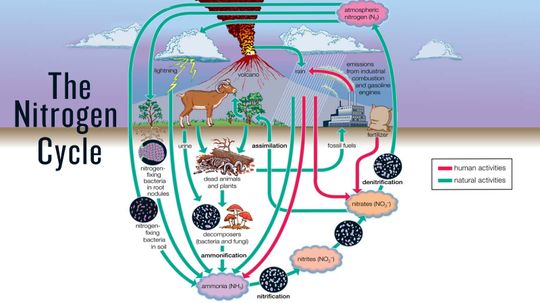
The nitrogen cycle is the system by which nitrogen is converted into different chemical forms, some usable to humans and animals and some not, as it circulates among the atmosphere, the land and the oceans.
Heat waves are becoming supercharged as the climate changes. How hot is too hot for normal daily activity, even for young, healthy adults?
By W. Larry Kenney, Daniel Vecellio, Rachel Cottle & S. Tony Wolf
Advertisement
Amanita phalloides is non-native to the North American continent, introduced to California from Europe, and rapidly spreading.
Distilleries call this evaporative substance "angel's share" and promise that it's not dangerous, but nearby residents find it coating everything around them and aren't so sure.
Cousins are indeed complicated. Who's your first cousin once removed? What is a second cousin? And what are kissing cousins? We'll tell you.
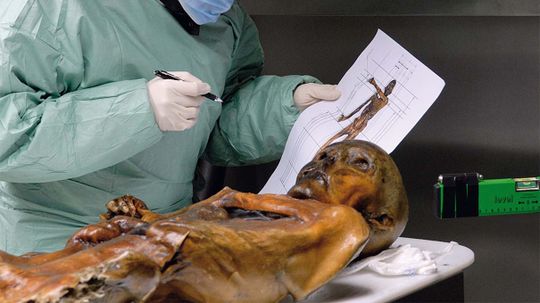
Researchers say that Otzi, the ancient man found in the Alps in 1991, lived on a diet loaded with fat to maintain warmth and energy in his cold, high-altitude environment.
Advertisement
Bezoars are concretions found in the stomachs of animals that were once believed to cure poisoning and plague.
By Loraine Fick
For years, speculation has surrounded the government's high security animal disease research center, which is slated to close in 2023.
Horseshoe crabs have blue blood that can detect toxins, a rare ability that's threatening their survival.
By Loraine Fick
A strange, but surprisingly accurate, ancient Egyptian pregnancy test survived for millennia and was spread around Africa and Europe because it was just that effective.