Energy Production
The greatest need modern civilizations have is energy. Learn about oil, electricity and newer forms of energy like solar and wind power.

The Fish Doorbell Isn't a Joke ... Seriously

The Euphrates River, at the 'Cradle of Civilization,' Is Drying Up

Study Says 2035 Is Climate Change Point of No Return

What State Has the Most Mountains in the U.S.? 8 Peak Records

The Most Dangerous Mountain to Climb (and 14 Giving Steep Competition)

15 Types of Gemstones to Add a Little Sparkle to Your Life

The Worst Air Quality in the World Is in Mountainous Terrain

The World Hits 8 Billion People; Is That Good or Bad?

Quiz: Can You Tell Climate Change Fact From Fiction?

6 Most Futuristic Cities Powered by Renewable Energy

Top 5 Green Robots

5 Things to Consider When Building a Solar-powered Home
Learn More
Wind energy is a cleaner alternative to fossil fuels, but people still ask: How many birds are killed by wind turbines?
By Zach Taras
As the global demand for clean energy intensifies, lithium has emerged as a critical player in the quest for sustainable technology. This invaluable resource, often dubbed "white gold," is essential for powering electric vehicles, renewable energy storage and advanced electronics.
Did you know that the sun shines more energy onto the Earth's surface than all of its inhabitants use in an entire year? Learn how to sell electricity back to the grid.
Advertisement
Wind farms are touted for their ability to capture a clean, renewable energy source. Is producing wind energy as beneficial as it seems, or are there any downsides?
By Lance Looper
Energy has been on everybody's minds lately, probably because our society is in a transition period, trying to move from polluting sources to cleaner ones.
Coal dominates the power industry in the U.S., producing nearly half of all electricity consumed in the country.
For most of the developed world, a flick of a switch brings the lights, television, computer, and dozens of other gadgets and appliances to life without question.
Advertisement
Shedding light on dark energy has been a bit of a challenge for today's astronomers. What dark energy actually is goes beyond our present scientific understanding.
By Talal Al-Khatib
The United States is the world's top producer of oil, but it still depends on foreign countries for millions of barrels, as well. Will there ever be a time when the U.S. is totally independent when it comes to oil production?
Why can't we generate all the electricity we need from the wind? Learn more about generating energy from wind power.
The cost of solar panels depends on how sunny it is where you live, how much you spend on electricity and what size PV system you need. Learn what factors into the cost of solar panels in this article.
Advertisement
The global oil supply can't meet the demand forever. Will the last drops of oil lead to widespread anarchy, the end of globalization and the relentless exploitation of previously protected drilling sites?
By Robert Lamb & Sascha Bos
Americans use a lot of gasoline -- hundreds of thousands of barrels each month, as a matter of fact. But did you ever wonder where all of that gas actually comes from?
The outer continental shelf of the United States could hold a sizeable amount of oil. But is it enough to make a difference in the price of oil?
Nuclear power stands as one of humanity's greatest scientific achievements, as well as one of the greatest risks to its self-extermination. This collection of images highlights some of the main features of nuclear power.
By Rick Mayda
Advertisement
Scientists working with Foster's Brewing Company have made a fuel cell using bacteria and the brewery's waste water. They claim that their fuel cell generates non-polluting power as it cleanses the water. But is the "beer battery" simply a novelty?
Where electricity is produced from a coal fired power station, how much coal is required to run a 100-watt light bulb 24 hours a day for one year?
You've probably never noticed how many of these you use everyday, but HowStuffWorks took the time to count them and take them apart to see what's inside!
Manhole covers have been blasting out of the ground in New York City. Find out what causes these strange explosions and how powerful they really are.
By Kevin Bonsor
Advertisement
The basic idea isn't new, but the process of modern hydropower conversion is high-tech. Today's hydropower plants are some of the coolest machines ever constructed. Find out how rushing water generates power.
By Kevin Bonsor
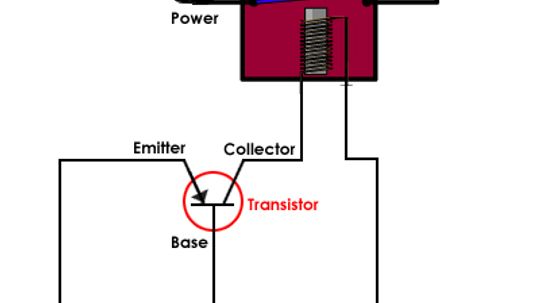
On the Flintstones, a small bird sits inside the light and turns it on every night before he goes to bed. In a modern streetlight, a small circuit replaces the bird.
Despite the dangers, oil refineries are essential to society in its current form. Learn how crude oil is converted into everything from butane to gasoline.
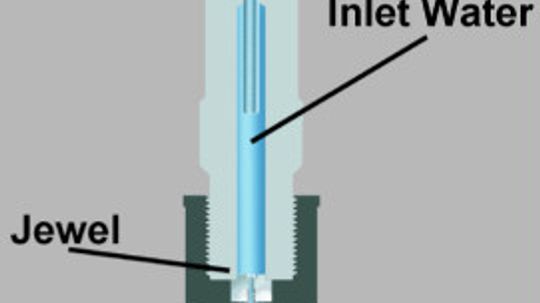
How can water cut through steel? A device called a waterjet uses extreme force to cut through all sorts of things.
Advertisement
OK, so volts measure the potential for energy to travel and ohms measure the resistance to the electrical flow, but what are amps and watts?
By Dave Roos
The Deepwater Horizon oil rig disaster has generated renewed interest in the way we search for oil. What methods do we use to find and extract this commodity from the Earth?