Nuclear Science
Nuclear science is the study of sub-atomic particles and their application in various disciplines. Here you can learn about nuclear power plants, atomic theory and radiation.
Brown Noise vs. White Noise: Which Is Best for Quality Sleep?

Can a sound wave kill you?

Can two cans and a string really be used to talk over a distance?

7 Types of Alcohol for Drinking, Cleaning and More

Understanding the Empirical Formula in Chemistry

The Most Expensive Metal in the World Isn't Gold or Platinum

How Electricity Works

How Faraday Cages Work

How Gasoline Works

What Does Mummification Have to Do With Gene Hackman?

What do bugs have to do with forensic science?

5 Things You Didn't Know About Autopsies

How Alchemy Paved the Way for Chemistry

How did Nikola Tesla change the way we use energy?

Time May Not Exist, Say Some Physicists and Philosophers

Why Does Ice Stick to Your Fingers?
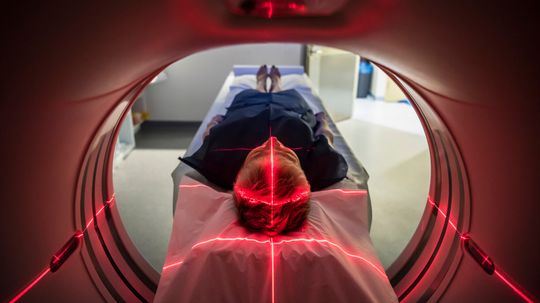
What if I forgot to remove a piercing before an MRI?
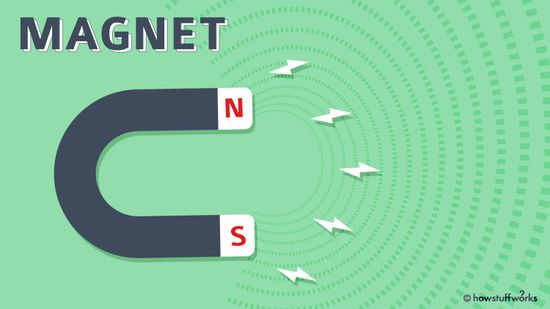
A Kid-friendly Introduction to Magnets and Magnetism
What's the Hardest Math Problem in the World? Try These 9

8 Types of Data That Inform Insights and Relationships
Congruent Angles: Definition, Symbol and Key Theorems

Tonnes vs. Tons: Metric vs. Imperial Measurements Strike Again

The Most Expensive Liquid Is 26,000x the Price of Human Blood

5 Hugely Fun Facts About Mass (Not Weight)

Why Are School Buses Yellow?

HowStuffWorks: How To Draw An Impossible Shape
What Are the Colors in the Visible Spectrum?
Learn More
In the history of atomic research, few stories are as gripping or cautionary as that of the demon core, a plutonium sphere designed for one of history's most devastating weapons. This tale not only encapsulates the highest point of atomic ambition but also serves as a somber reminder of the human cost associated with such power.
In nuclear physics, the concept of half-life plays a crucial role in understanding the decay of radioactive substances. Scientists use the half-life formula in other disciplines to predict the rate of decay, as well as measure the age of ancient artifacts through carbon dating.
By Yara Simón
The Standard Model of physics provides a framework for the subatomic world of all energies. Could a possible newfound carrier boson expand the definition of that framework?
By Mark Mancini
Advertisement
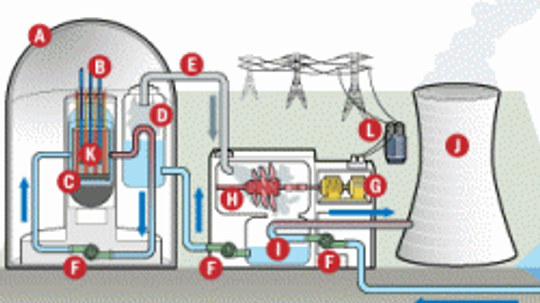
Tour the inside of a nuclear power plant with these illustrative diagrams to learn more about how nuclear power plants work.
The nuclear arms race was a frantic era in which several nations tested nuclear technology and stockpiled warheads. Read about the nuclear arms race.
By John Fuller
Dropping atomic bombs on Hiroshima and Nagasaki ended World War II. How did the most powerful weapon in the world get developed? It started with the Manhattan Project.
By John Fuller
Nuclear materials get used in many forms of nuclear medicine -- everything from PET scans to chemotherapy uses them. Learn how nuclear medicine works.
Advertisement
On the one hand, nuclear power offers a clean energy alternative that decreases fossil fuel dependence. On the other, it summons images of quake-ruptured Japanese power plants leaking radioactive water. What happens in reactors in good times and bad?
Iran has announced its activation of a second set of uranium centrifuges. These machines are at the core of the uranium-enrichment process. Find out where the centrifuge fits into the equation.
If the sight of a mushroom cloud burning above the horizon suggests that the nuclear weapon-equipped world might end with a bang, then nuclear winter presents the notion that post-World War III humanity might very well die with a whimper.
By Robert Lamb
In the comics, radiation exposure turned an average man into a pea green and angry Incredible Hulk. But in reality, what can radiation do to those exposed? Is it always a villain?
By Debra Ronca
Advertisement
When the power goes out and is later restored, how do you know what time to set your clocks to? Have you ever wondered how time is regulated? Learn how scientists determine exact time.
Nuclear radiation can be extremely beneficial or extremely harmful -- it all depends on how it's used. Learn what nuclear radiation is all about.
I once saw this device shaped like a light bulb. It had a vertical support inside it, and on that support there were four vanes with four diamonds on the end. One side of the diamond was black and the other was white. I did a little research and found out that it was called a Crookes' radiometer -- how does it work?
Many ads for new clocks advertise their ability to automatically synchronize themselves with the atomic clock in Boulder, Colorado. This atomic clock is more precise because it uses the frequencies of atoms as its resonator.
Advertisement
Fusion reactors will use abundant sources of fuel, will not leak radiation above normal background levels, and will produce less radioactive waste than current fission reactors. Learn about this promising power source.
By Patrick J. Kiger & Craig C. Freudenrich
Atom smashers tell us about the fundamental structure of matter, the forces holding it together and the origins of the universe. Discover how scientists use particle accelerators to break atoms apart to learn about the nature of reality.
Thanks to our voracious appetite for energy, the element long linked with nuclear weapons is taking on a new role. Where does the hunt begin for uranium?
Explosions, fires and dangerous radiation levels dominated the headlines after the March 11 earthquake and tsunami sparked a nuclear crisis in Japan. How did so many safety measures fail?
Advertisement
First discovered in the late 1930s, muons are passing through you and everything around you at a speed close to light, as cosmic rays strike particles in our planet's atmosphere. So what are muons and how are they informing the new physics?
The lava-like material that formed after the Chernobyl nuclear disaster is a deadly example of corium, a hazardous material created only after core meltdowns. Five minutes next to it can kill a human.
Thorium is in many ways safer than uranium for nuclear power production. But is it safe enough to bet on for our energy future?
The seriously ambitious experiment aims to understand the mysterious neutrino and maybe even figure out why matter won out over antimatter during the Big Bang.
Advertisement
The Large Hadron Collider isn't just a one-trick (Higgs) pony. Find out what else has happened where hundreds of millions of particles may collide any given second.
When something as important as the Higgs rocks our world, we want to know every last thing about it, including what it looks like. So?