Explosives
Get the science behind the inner workings of bombs, missiles and handheld weapons, including grenades. Learn how they work and how they are used to inflict maximum damage.

Watch Your Six: Military Jet Pictures

McDonnell Douglas F-4 Phantom II

Lockheed P-38 Lightning

Does Army experience help your civilian career?

How NCO Professional Development Ribbons Work

How Army Reconnaissance Jobs Work

How Agent Orange Worked

How Biological and Chemical Warfare Works

How Mustard Gas Works

What Is the Strongest Military in the World?

5 Countries That Ditched Their Military Forces

Coast Guard Rescue Swimmers Risk All to Save Lives

HowStuffWorks Illustrated: Two Legal Gun Modifications

Gun Pictures

What's the world's smallest gun?

Are robots replacing human soldiers?

Can drones replace fighter jets?
Do wars drive technological advancement?

Submarine Pictures

How the Zumwalt Class Destroyer Works

How Aircraft Carriers Work
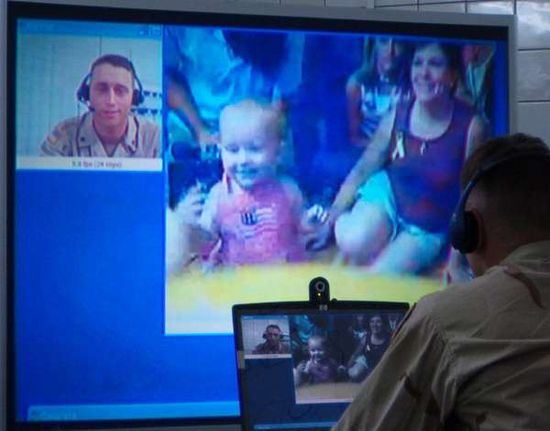
How Military Video Conferencing Works

Why a Draft Would Weaken the U.S. Military

What Was the First War?

Top 5 Gadgets on the High-tech Soldier

Ghillie: Fishing Aid and Inspiration for Camo Suits

10 Insane Disguises That Actually Worked

How Code Breakers Work

YOU Can Drive a Tank!

Is the army testing an invisible tank?

Centurion Main Battle Tank
Learn More
An underwater explosion or UNDEX won't absorb the destruction like air does. Learn why an underwater explosion could harm you more than one on land.
By Robert Lamb
"Die Hard" films' John McClane (not to mention a long line of other Hollywood action heroes) never met an explosion he couldn't outrun. In real life, it's not so easy to sprint away from a blast.
By Chris Opfer
Grenades are a devastatingly effective weapon on the battlefield. Find out what happens when a soldier pulls the pin and tosses one of these miniature bombs at the enemy.
By Tom Harris
Advertisement
The Stinger missile is a deadly man-portable air-defense system (MANPADS) that can be rapidly deployed by ground troops. It's lightweight, combat-proven and has a greater than 90 percent success rate. So how are Stingers used and against whom?
C-4 is in the news quite a bit these days -- it's a powerful explosive that's used in terrorist attacks all over the world. Find out what C-4 explosive is and what it can do.
By Tom Harris
A Sidewinder missile weaves through the air toward an enemy target as if it has a mind of its own -- and in a way, it does. High-tech "smart weapons" take most of the guess work out of hitting a target. Find out how Sidewinders seek and destroy.
By Tom Harris
Landmines are a deadly legacy of 20th century warfare. Independent sources report that since 1975, landmines have killed or maimed more than 1 million people during peace time. Learn about the technology of landmines and their deactivation.
By Kevin Bonsor
Advertisement
Nine countries hold the 13,000 nuclear weapons in the global stockpile. That's less than during the Cold War but it doesn't change the fact that these bombs are still a threat to global humanity. So how do they work and are we close to nuclear war?
What once was "Star Wars" under Reagan is now National Missile Defense under Bush. Learn more about the technology behind the system.
By Kevin Bonsor
Your most rugged pair of blue jeans can't hold a candle to the cutting-edge blast-resistant clothing and technology. Sure, these fabrics are tough, but can they diffuse bomb blasts?
By Tom Scheve
Those same, buzzing insects that seek out molecular hints of the pollen they use to make honey can just as easily detect traces of materials used to bombs. How are honeybees used to find bombs?
By Julia Layton
Advertisement
The Kim Jong Un regime continues to demonstrate its desire to threaten the U.S. and its allies with nuclear-armed ICBMs. But can any of these missiles actually reach the U.S. mainland?
By Julia Layton & Sarah Gleim
In the first reports released since North Korea announced its underground nuclear test on Monday, officials are saying they have found no evidence of a nuclear signature in the air above the blast site.
By Julia Layton
Whether you call it a homemade bomb, a booby trap or an improvised explosive device, an IED is simple to make, easily hidden and extraordinarily destructive. Why are these deadly devices one of the No. 1 killers of soldiers in Iraq.
Suicide bombings are chillingly logical. By hiding explosives on a willing carrier, individuals smuggle death into densely populated areas. But are these bombers strictly a modern phenomenon?
By Robert Lamb
Advertisement
You may have thought militaries stopped using napalm after the Vietnam War thanks to the United Nations, but this incendiary weapon lives on in modern warfare. Has it also been used in Iraq?
In what may prove to be the first nuclear explosion since 1998, North Korea claims it has conducted an underground test of a nuclear weapon.
By Julia Layton
A fascinating article that describes how cruise missiles work and explores some of their advanced technology!
When the U.S. Air Force tested the MOAB last year, it tested one of the largest conventional bombs ever built. MOAB stands for Massive Ordnance Air Burst, and it's not for the faint-hearted. Find out how it fits into the U.S. arsenal.
Advertisement
Smart bombs can navigate their own way to specific ground targets with startling accuracy, even in poor weather conditions. Find out how JDAMs and other smart bombs automatically find and hit their targets.
By Tom Harris
Learn how EMPs work and how these weapons could tear apart modern technology.
By Tom Harris
The U.S. Pentagon says it is sending two Patriot missile batteries to Poland to help NATO add to its defense strategy against a possible Russian attack. How do Patriot missiles work?
Ordinary bombs can take out surface facilities; but when the target is underground or otherwise embedded, the job requires a bomb with penetrating power. That's where bunker busters come in.
Advertisement
Rugged and simple, suitable for downing helicopters, disabling tanks or attacking buildings at close range -- in the hands of a skilled operator, the RPG is a lethal and versatile weapon.
By Shane Speck
Bombs come in many different shapes and sizes -- as small as a grenade to as huge as a thermonuclear warhead. See what the inside of a bomb looks like and learn how it's detonated.























